The analysis of intact proteins is now an established technique for many aspects of biologics testing such as the gross comparison of biolsimilars, determination of molecular mass, glycoform analysis, antibody drug conjugate (ADC) ratios and for the determination of post-translational modifications (PTMs). Many aspects of in-tact (or top-down) protein analysis have been facilitated through improvements in both sensitivity and resolution of mass spectrometric detectors, however, to take full advantage of the benefits of this approach, one needs to carefully consider the chromatographic mode as well the chemical and physical characteristics of the stationary phase material. Here we consider selected reversed phase HPLC applications from our key suppliers, to highlight the possibilities of successful intact protein analysis and the critical variables to consider for the best analytical outcomes.
Stationary Phase Support Characteristics
Successful intact analysis of large proteins such as monoclonal antibodies (mAb) requires the careful consideration of several factors such as the stationary phase pore size and stationary phase alkyl chain length both of which are related the molecular weight of the analyte(s), the mode of detection to be employed, mobile phase conditions and the required throughput of the final method.
Traditional reversed phase HPLC stationary phase particles with 100 or 120A pores are not suitable for the analysis of large proteins which may be excluded from the pore structure and both silica and polymer-based phases of 300, 450 or 1000A are now available to successfully analyse intact proteins of higher molecular weight. This phenomenon is highlighted in Figure 1 using HALO superficially porous stationary phase and a competitor phase of 400 and 200A pore size respectively.
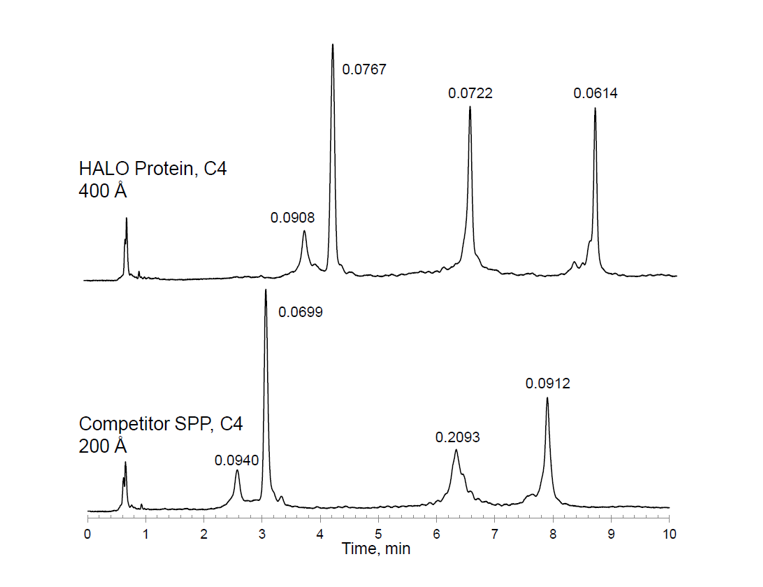

Peak Identities (in order):
1. Catalase, 250 kDa
2. α-Chymotrypsinogen A, 25.0 kDa
3. β-Galactosidase, 465 kDa
4. β-Amylase, 200 kDa
Figure 1. Effect of stationary phase pore size on the separation of in-tact proteins
Conditions
| Columns | 2.1 x 100 mm HALO Protein C4 (400A) and Competitor SPP C4 (200A) |
| Injection Volume | 1 μL |
| Detection | UV 280 nm |
| Temperature | 60 oC |
| Mobile Phase A | water/0.1% TFA |
| Mobile Phase B | 80/20 ACN/water/0.1% TFA |
| Gradient | 40-47% ACN in 10 min. |
| Flow rate | 0.3 mL/min |
As can be seen from Figure 1, peak shape and efficiency (peak widths at half height are shown above each peak in the chromatogram) are much improved when using the larger diameter pore size support due to increased accessibility to the pore structure and therefore increased available surface area.
Figure 1 also highlights an important aspect of in-tact protein analysis in terms of the very high ‘sensitivity co-efficient’ of macromolecules relative to smaller analytes, which drives the requirement for much shallower eluotropic strength gradients to achieve analyte separation. In this case a change of only 7%B in 10 minutes is enough to achieve a satisfactory separation. This is especially important when analysing closely related species such as isoforms or post translational modifications as we will see in a later example.
Pore accessibility and the high efficiency of core-shell particles is also highlighted in Figure 2 in which the Agilent AdvanceBio RP-mAb C4 3.5mm column with 450Å pores is compared to other phases with different pore sizes. This column is specifically designed and optimised for the analysis of intact and fragment analysis of monoclonal antibodies.


Figure 2. Effects of pore and particle size on the analysis of intact monoclonal antibody (intact humanized recombinant Herceptin IgG1)
Conditions
| Columns | Agilent AdvanceBio RP-mAb C4 2.1 x 100 mm, 3.5 µm (450Å) |
| Mobile phase | (A) 0.1% TFA in water:IPA (98:2) (B) IPA:ACN:Mobile phase A (70:20:10) |
| Flow rate | 1.0 mL/min |
| Gradient | 10-58% B in 4 min, 1 min wash at 95% B, 1 min re-equilibration at 10% B |
| Sample | 5 μL injection of humanized recombinant Herceptin Variant IgG1 intact from Creative Biolabs (1 mg/mL) |
| Temperature | 80 °C |
| Detection | UV @ 254 nm |
It is clear from Figure 2 that the AdvancedBio RP-mAb C4 column provides superior peak shape and resolution to those columns with lower pore size.
HALO Bioclass superficially porous particles from Advanced Materials Technology (AMT) are also available in 1000A pore size for successful analysis of increasingly high molecular weight proteins, as shown in Figure 3. It is interesting to note that improved efficiency is also derived through the use of superficially porous substrates versus the fully porous equivalent, due to reduction of fixed rate diffusion through the ‘shallower’ porous shell (i.e. reduced diffusion path lengths).
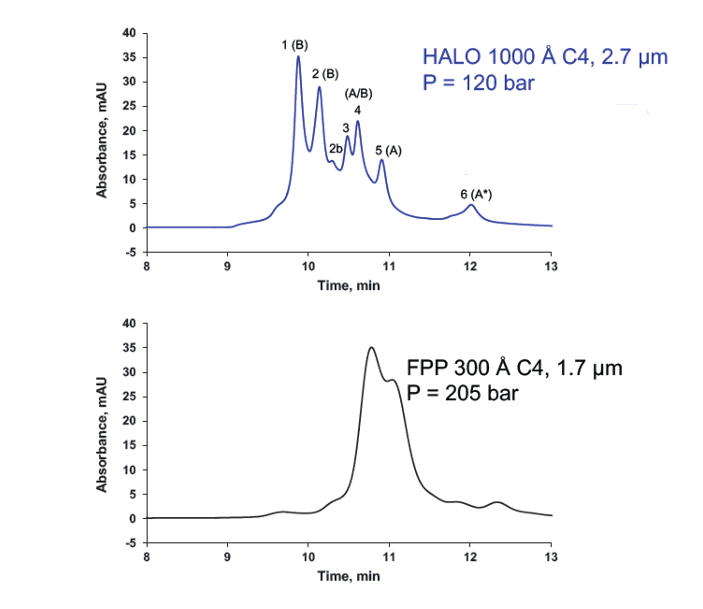

Peak Identities (in order):
1. IgG2-B
2. IgG2-B
2b. IgG2-B
3. IgG2-A/B
4. IgG2-A/B
5. IgG2-A
6. IgG2-A*
Figure 3. The large pores of the HALO 1000 Å C4 column allow improved access to the stationary phase and increased resolution for IgG2 isoforms compared to the smaller 300 Å pores of the fully porous C4 column
Conditions
| Columns | HALO 1000 Å C4, 2.7 µm, 2.1 x 150 mm |
| Mobile phase | (A) 88/10/2 water/ACN/n-propanol/0.1% DFA (B) 70/20/10 n-propanol/ACN/water/0.1% DFA |
| Flow rate | 0.2 mL/min Temperature: 80 °C |
| Gradient | 14-24% B in 20 min |
| Injection | 2 µL |
| Detection | 280 nm, PDA |
| LC System | Shimadzu Nexera |
Whilst it is not possible to be definitive in the correlation between stationary phase pore size and protein molecular weight, the following table from Advanced Materials Technology gives a good guide to superficially porous stationary phase particle characteristics for various analyte classes ;
| COLUMN NAME | PORE SIZE(S) (um) | PARTICLE SIZE(S) (um) | SURFACE AREA (m2/g) | STATIONARY PHASES | TARGET ANALYTES |
| HALO Peptide | 160 | 2, 2.7, 5 | 65, 90, 60 | ES-C18, ES-CN, Phenyl-Hexyl |
Peptides and polypeptides 100 Da < MW < 15 kDa |
| HALO Protein | 400 | 3.4 | 15 | C4, ES-C18 |
Peptides, polypeptides, and proteins 2 kDa < MW < 500 kDa |
| HALO Protein | 1000 | 2.7 | 22 | C4, ES-C18, Diphenyl |
Large proteins, mAbs, mAb fragments, and ADCs > 50 kDa |
The ability of superficially porous particles to overcome the effects of slower diffusion kinetics and enhance the efficiency of large molecule separations can be used to achieve faster separations. Figure 4 shows the use of HALO 3.4mm, 400A superficially porous particles to produce an equivalent separation of reference proteins in around one of third of the analysis time compared to a fully porous 1.7mm, 300A material. It should also be noted that the back pressure obtained is almost identical for both separations.
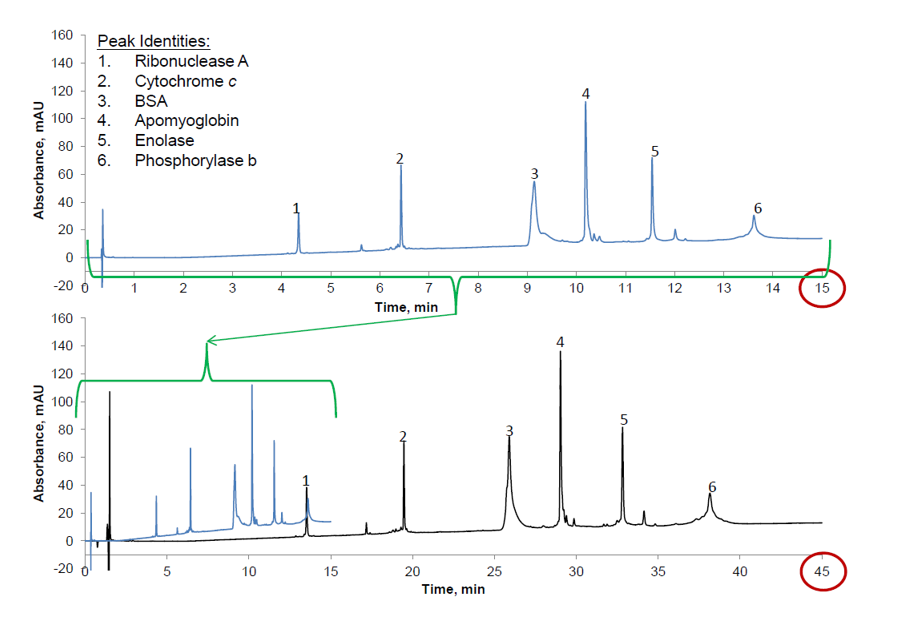

Figure 4. Superficially particles with larger particle and pore size allows faster eluent flow rates to achieve equivalent separation with the same system back pressure in around one third of the time compared to a sub 2mm particle with smaller pore size
Conditions
| Columns | HALO Protein C4, 400 Å, 3.4 µm |
| P | 132 bar |
| Mobile phase | (A) water/0.1% TFA (B) Acetone/0.1% TFA |
| Flow rate | 0.6 mL/min |
| Gradient | 20-60% B in 15 min |
| Temperature | 60 °C |
| Columns | Totally porous, 300 Å, C4,1.7 µm |
| P | 134 bar |
| Mobile phase | (A) water/0.1% TFA (B) Acetone/0.1% TFA |
| Flow rate |
0.2 mL/min
|
| Gradient | 20-60% B in 45 min. |
| Temperature | 60 °C |
The Agilent AdvanceBio RP-mAb column is also a superficially porous particle with wider rope diameter and can deliver higher resolution and faster run times to provide accurate, reproducible results when analysing monoclonal antibodies for biopharma discovery, development, and QA/QC applications. Figure 5 depicts the optimised RP HPLC elution profile of intact innovator and biosimilar rituximab on an AdvanceBio RP-mAb C4 stationary phase and reduced column dimensions (2.1 × 50 mm, 3.5 μm, 450A) to produce excellent shape with a total run time of only 4 minutes.
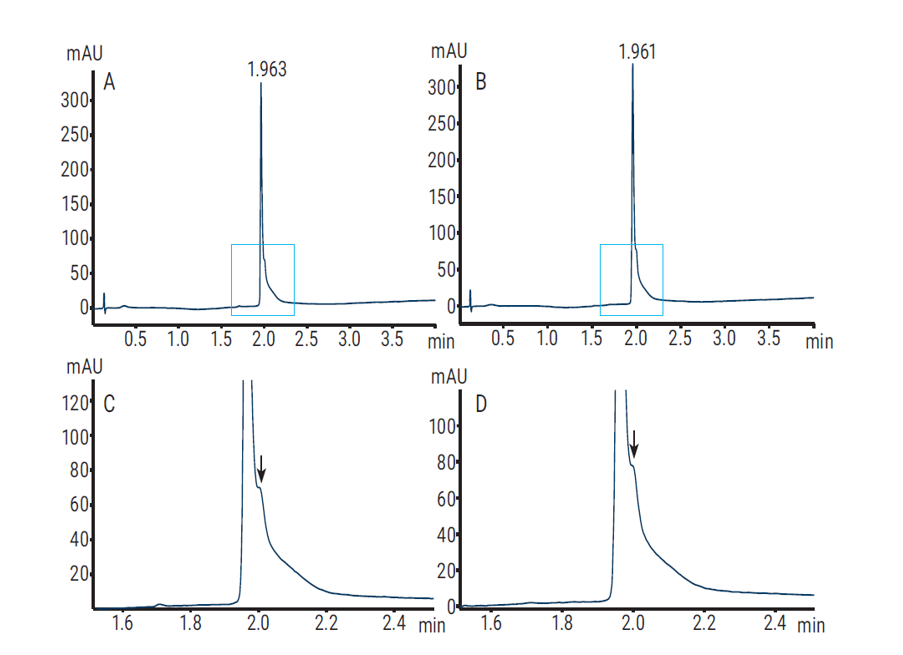

Figure 5. RP-HPLC profile of intact innovator rituximab (A) and biosimilar rituximab (B) on an Agilent AdvanceBio RP-mAb C4, 2.1 × 50 mm, 3.5 μm column. C and D show zooms
Conditions
| Columns | Agilent AdvanceBio RP-mAb C4, 2.1 × 50 mm, 3.5 μm, 450A |
| Mobile phase |
A) Water + 0.1 % TFA
B) IPA:ACN:Water (70:20:10) + 0.09 % TFA |
| Flow rate | 1.0 mL/min |
| Gradient | 0 – 60% B in 4 minutes |
| Injection | 1 μL |
| Detection | UV @ 220 and 280 nm |
| Temperature | 80 °C |
| Post time | 2 minutes |
YMC Triart is an organic / inorganic silica hybrid particle which is triple end-capped to achieve very low secondary interactions and therefore improved peak shape. Triart particles are available in 1.9mm diameter with 300A pores, which is an optimised balance for fully porous silica materials and can achieve in-tact protein retention and separation as is shown in Figure 6 with excellent peak shapes achieved for commercially available protein therapeutics.


Figure 6. YMC Triart C4 fully porous particles with a balanced particle and pore size are used to successfully retain several commercially available protein therapeutics with excellent peak shape
Conditions
| Columns | |
| Mobile phase | A) water/TFA (100/0.1) B) acetonitrile/TFA (100/0.1) |
| Flow rate | 0.4 mL/min |
| Gradient | 25-45%B (0-10 min) Detection: UV at 280 nm (0.13s, 40Hz) |
| Injection | 2 μL |
| Sample conc. | 0.5 mg/mL |
| Temperature | 80 °C |
The combination of optimised support particle morphology, with reduced column length and internal diameter allow rapid characterisation of in-tact proteins for screening and critical quality attribute determination, using the YMC Triart Bio C4 hybrid particle technology.
Bonded Phase Characteristics
In order to reduce the detrimental effects of slower mass transfer kinetics associated with increasing molecular weight of the protein analyte species, shorter alkyl chain species or non-alkyl stationary phase chemistries can be used, leading to improvements in the efficiency of the chromatographic separation. It is also always useful to have alternatively selectivity’s within the chromatographers armoury and stationary phases such as Diphenyl are useful in this regard.
Figure 7 below demonstrates these principles using the Agilent AdvanceBio RP-mAb superficially porous particle with C4, C8 and diphenyl bonded phase chemistries. It is clear that all three chemistries offer differing retentivity and that the diphenyl phase shows clear selectivity differences from the alkyl based phases for IgG1 intact Trastuzumab. This can be very useful when investigating minor structural variants, isoforms, impurities etc.


Figure 7. Influence of different bonding chemistries on the retention and selectivity of IgG1 Trastuzumab (Herceptin) chromatograms using superficially porous Agilent AdvanceBio RP-mAb columns
Conditions
| Columns | |
| Mobile phase | A) 0.1% TFA in water:IPA (98:2) B) IPA:acetonitrile:MPA* (70:20:10) |
| Flow rate | 1.0 mL/min |
| Gradient | 10-58% B in 4 min, 1 min wash at 95% B, 1 min re-equilibration at 10% B |
| Detection | UV @ 254 nm |
| Sample | 5 μL injection of Humanized Recombinant Herceptin Variant IgG1 Intact from Creative Biolabs (1 mg/mL) |
| Temperature | 80 °C |
When choosing a stationary phase, it is important to start with the chemical characteristics of your target molecule and its impurities. This understanding will help to leverage your phase choice for optimal results. As a general rule, the table below acts as a guide for selectivity, but as best practice, you should screen several different bonded phase chemistries in order to fully investigate the selectivity space for your analytes of interest.
Select the right bonded phase for optimal results
C4: Best for fast, high-resolution separations, hydrophobic proteins
SB-C8: Fast, high-resolution separations for less hydrophobic proteins, increased resolution between closely
related impurities (similar hydrophobicity profiles)
Diphenyl: Orthogonal selectivity (confirmational), or when separating species based on aromatic or pi-pi properties
There are also currently three bonded phases available on HALO 1000 Å Fused-Core® particles – C4, ES-C18, and Diphenyl. Each shows unique selectivity for the separation of monoclonal antibodies. In Figure 9 denosumab isoforms are resolved using a shallow gradient with the addition of n-propanol. Diphenyl phase is the most retentive phase, followed by ES-C18, and then C4. All three phases are recommended to be screened to determine which one yields the optimum separation for mAbs under investigation.


Peak Identities (in order):
1. IgG2-B
2. IgG2-B
3. IgG2-A/B
4. IgG2-A/B
5. IgG2-A
6. IgG2-A*
Disulfide bridge isoforms of IgG2
Note retention order is equivalent for all stationary phases
Figure 8. Influence of different bonding chemistries on the retention and selectivity of IgG1 denosumab chromatograms using superficially porous HALO 1000 Å Fused-Core® particles
Conditions
| Columns | |
| Mobile phase | A) 2/10/88 n-propanol/ACN/H2O + 0.1% DFA B) 70/20/10 n-propanol/ACN/H2O + 0.1% DFA |
| Flow rate | 0.2 mL/min |
| Gradient | 16-26% B in 20 min |
| Detection | 280 nm, PDA; 350 nm reference |
| Injection | 2.0 μL of 2 mg/mL denosumab |
| Sample Solvent | Water (0.1% TFA) |
| Temperature | 80 °C |
Molecular weight determination using high resolution mass spectrometry of intact proteins is a common application. Agilent AdvanceBio RP-mAb superficially porous columns produce very high efficiency, fast chromatography. The ability to cross compare molecular weight measurements using different stationary phase chemistries provides confirmational data. Figure 9 shows the results of such an analysis using C4 and complimentary Diphenyl phase which resulted in molecular weight confirmation.


Figure 9. MS analysis of intact mAbs – excellent peak shape, fast chromatography allows molecular weight determination
Conditions
| Columns | |
| Mobile phase | A) 0.1% FA in water B) IPA:ACN:water (80:10:9.9) + 0.1% FA |
| Flow rate | 0.6 mL/min |
| Gradient | 0 min, 20% B; 4 min, 20% B; 5 min, 40% B; 10 min, 70% B; 11 min, 90% B; 11.1 min, 20% B |
| Detection | Agilent 6530 Accurate-Mass Q-TOF LC/MS |
| Injection | 1 μL (1 μg/μL) |
| Sample | Innovator Herceptin |
| Temperature | 80 °C |
For those who do not have access to high resolution mass spectrometry, approximate molecular weight determination may be made using a diol phase which can be calibrated with a series of proteins of known molecular weight UV detection . This is demonstrated in Figure 10 using a YMC pack Diol column. The Diol 120A, Diol-200A and Diol-300A are suitable for the separation and molecular weight determination of proteins with molecular weights from 1000 to 1,000,000 Da.


| Protein Calibrant | Molecular Weight |
| 1. Glutamate dehydrogenase | 290,000 |
| 2. Lactate dehydrogenase | 142,000 |
| 3. Enolase | 67,000 |
| 4. Adenylate kinase | 32,000 |
| 5. Cytochrome C | 12,400 |
Figure 10. Silica based diol columns can be used for molecular weight determination through retention time versus molecular weight of a series of well characterised proteins
Conditions
| Columns | |
| Mobile phase | 0.1M KH2PO4.K2HPO4 (pH 7.0) with 0.2M NaCl |
| Flow rate | 0.7mL/min. |
| Detection | UV at 280 nm |
| Temperature | Ambient |
Non-silica stationary phases
PLRP-S is a polymeric particle which gives a typical reversed-phase separation, although with somewhat different selectivity and the advantage of wide pH tolerance, and a wide variety of pore sizes. While substantially larger than is commonly used for intact proteins, the 1000 Å, 5 μm PLRP-S columns give excellent results for intact protein and protein fragment analysis.
The correct choice of LC column and method is critical to achieve reproducible high-resolution separations and high‑quality MS data. Typically, using formic acid (FA), an MS-friendly ion-pairing agent, in the mobile phase leads to poor total ion chromatogram (TIC) peak shape with traditional silica‑based reversed-phase columns. This affects LC/MS results (resolution, sensitivity, MS signal, accurate molecular weight information, and so forth). Hence, there is a critical requirement for an LC column compatible with formic acid conditions for enhanced LC/MS analysis of biomolecules. Polymeric and resin based columns are typically resistant to the lower pH associated with the use of formic acid as a mobile phase additive.
Figure 11 uses an Agilent PLRP-S to demonstrate the LC/MS analysis of mAbs and anti-body drug conjugates (ADC). PLRP-S columns contain rigid, macroporous, spherical particles of polystyrene and divinylbenzene. They are physically and chemically stable across the complete pH range. The particles are inherently hydrophobic, therefore, neither bonded phases nor alkyl ligands are required for reversed-phase separations. This gives a highly reproducible material that is free from silanols and heavy metal ions which results in excellent peak shape.


Figure 11. Intact mAb/ADC LC/MS analysis on an Agilent PLRP-S, 2.1 × 50 mm, 5 μm, 1,000Å column. Top: Total ion chromatogram; bottom: Deconvoluted spectrum. FWHM: full width at half maximum.
Conditions
| Columns | |
| Mobile phase | A) 0.1% FA in water B) 0.1% FA in ACN |
| Flow rate | 0.6 mL/min |
| Gradient | 20% to 70% B in 10.1 mins. |
| Injection | 1 μL |
| Sample Thermostat | 5 °C |
| Temperature | 80 °C |
The PL-RPS column provides excellent TIC peak shapes with ≤ 0.1 minutes full width at half maximum (FWHM) mAbs and 0.25 minutes FWHM ADC. The narrow TIC peak width was obtained using a standard RP mobile phase system (ACN + FA). Similar peak widths were observed for two mAbs, demonstrating the suitability of the method for various mAb samples. Narrow peak width was also demonstrated with the ADC sample, which is highly heterogeneous
The Thermo MAbPac column contains a resin particle based on hydrophobic polystyrene divinylbenzene supermacroporous 4μm polymer particles, designed for use in reversed phase mode. These columns are again stable in the pH range 1-14 and at temperatures up to 110oC, which can present significant advantages as peak efficiency of macromolecules typically increases with increasing temperature. The eluent pH range flexibility gives the option to investigate the full range of mobile phase additives to optimise both resolution and MS detection sensitivity.
Figure 12 shows an interesting application in which various glycoforms of Trastuzumab are investigated from the high-resolution mass envelope of the intact molecule under denaturing reversed phase conditions. Further information on glycoform analysis will be presented in future editions in this series on biomolecule chromatography.
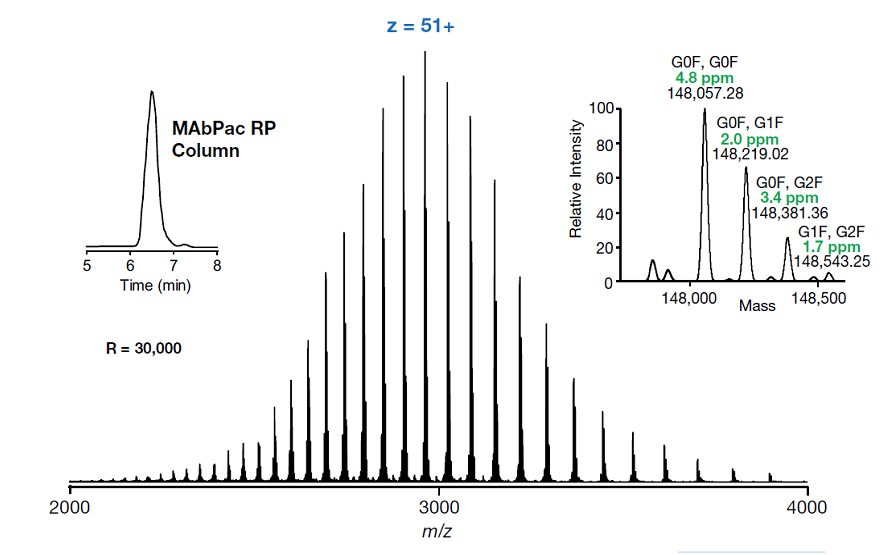

Figure 12. Intact glycoform analysis of Trastuzumab using the resin based THermo MAbPac column with 1500A pores
Conditions
| Columns | |
| Mobile phase | A) Water with 0.1% formic acid (v/v) B) Acetonitrile with 0.1% formic acid (v/v) |
| Flow rate | 250 µL/min |
| Gradient | 25 – 80 %B in 10 minutes |
| Temperature | 80 °C |
The non-functionalised, wide pore resin based column produces an efficient peak for the intact trastuzumab protein with good peak shape and, through the use of formic acid as the eluent modifier, results in good sensitivity of the MS signal. In combination, these factors allow successful glycoform analysis of the intact protein.
Silica and Hybrid Columns with pH and temperature resistance
We have seen in the previous section how important it is to be able to manipulate both temperature and eluent pH (via the use of different modifiers) in order to optimise the efficiency, selectivity and sensitivity of macromolecule separations.
This illustrated in Figure 13 in which Trastuzumab (Herceptin) is chromatographed using different eluent additives, which have different ion pairing strengths and result in different eluent pH.
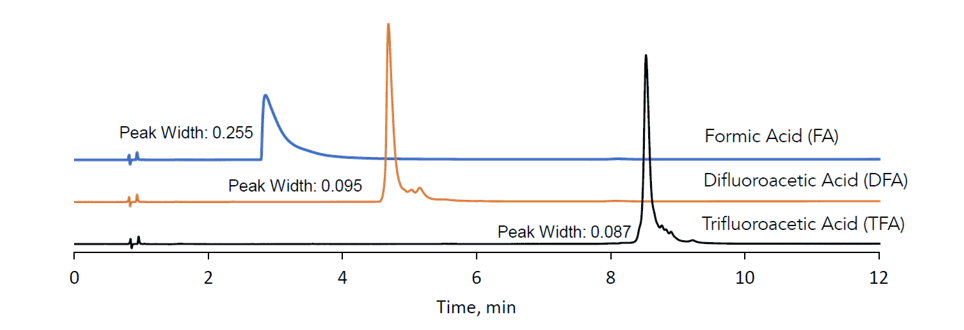

Figure 13. Intact analysis of Trastuzumab using different eluent additives on the HAL) 1000A C4 column
Conditions
| Columns | |
| Flow rate | 0.4 mL/min |
| Gradient | 35-47.5% AcN/0.1% acid as indicated in 12 min |
| Sample | 2 μL of trastuzumab - 2 μg/μL (30/70 ACN/H2O) |
| Temperature | 80 °C |
Figure 13 shows that either TFA or DFA can be used as mobile phase additives instead of formic acid to provide much narrower and more symmetrical peaks, and to allow adjustments to retention and resolution among minor variants. This orthogonal selectivity allows exploration of minor structural variants and ensures important analytical information is not missed. Despite the HALO phase being silica based, it shows good stability under acidic conditions and can also be used at higher temperatures, as shown in Figure 14.
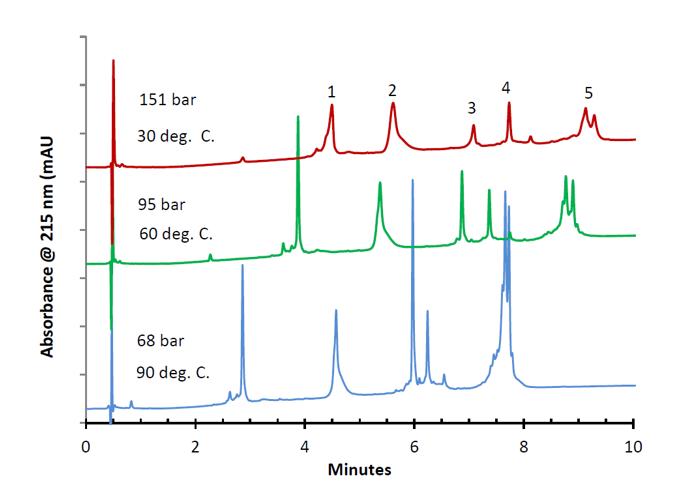

Peak Identities (in order):
1. Lysozyme 14.3 kDa
2. BSA 66.4 kDa
3. α-Chymotrypsinogen A 25.0 kDa
4. Enolase 46.7 kDa
5. Ovalbumin 44.0 kDa
Figure 14. Intact analysis of various proteins on the HALO 400A C4 column, highlighting the improvement in peak efficiency and changes in selectivity at higher temperatures
Conditions
| Columns | |
| Instrument |
Agilent 1200 SL Injection Volume: 2 µL Detection: 215 nm
|
| Mobile phase |
A) water/0.1% TFA
B) acetonitrile/0.1% TFA |
| Flow rate | 0.45 mL/min |
| Gradient | 28-58% B in 10 min. |
| Temperature | as indicated |
These separations demonstrate the effect of elevated temperatures on the efficiency of protein separations under reversed-phase conditions on a HALO Protein C4, 3.4 μm, 400Å pore column. One observes higher signal to noise and narrower peaks as the temperature increases. The HALO C4 phase has been shown to be very stable even at these elevated temperatures.
YMC-Triart Bio C4 is an organic/inorganic hybrid silica based wide-pore column suitable for the separation of proteins with molecular weight of up to 150,000, such as antibodies, under high temperature conditions. YMC-Triart Bio C4 achieves excellent peak shape even when using mobile phase containing formic acid and is therefore effective for high sensitivity LC/MS analysis. The phase is also useful for separation of peptides, oligonucleotides, and small molecules with high hydrophobicity which are difficult to separate using C18 columns.
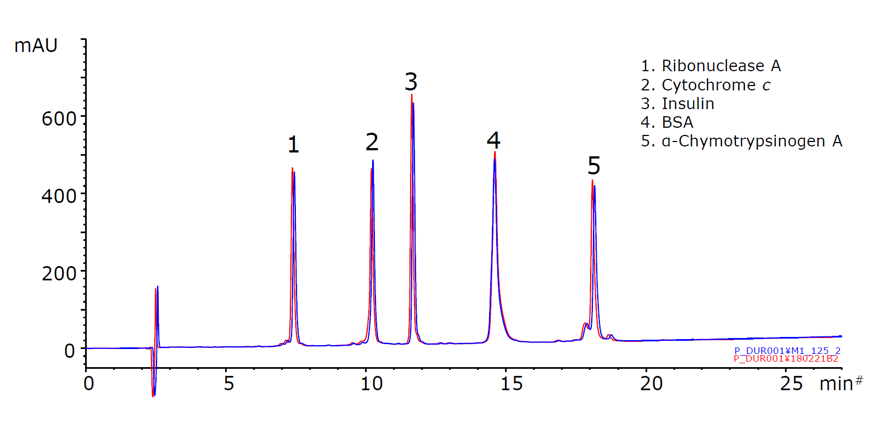

Figure 15. YMC-Triart Bio C4 hybrid particles shows high chemical in 0.1% TFA at 70oC – Blue: Initial Separation Red: 3000 Column Volumes (125 hours, 150 Injections)
Conditions
| Columns |
YMC-Triart Bio C4 (5 μm, 300 Å), 150 x 3.0 mm ID
|
| Mobile phase |
A) water/TFA (100/0.1)
B) acetonitrile/TFA (100/0.1) |
| Flow rate | 0.4 mL/min |
| Gradient | 20-60%B (0-27 min), 90%B (27-35 min), 20%B (35-50 min) |
| Detection | UV at 220 nm |
| Injection | 10 μL (0.25 ~ 0.50 mg/mL) |
| Temperature | 70°C |
Figure 15 demonstrates no change in retention time and peak shape is observed when using YMC-Triart Bio C4 under chemically harsh conditions such as the strongly acidic eluent and high temperature. This excellent chemical stability presents the chromatographer with the full range of options to explore the entire selectivity space when analysing intact proteins.
Generally, improvement of protein separations is expected under high temperature conditions using a mobile phase containing TFA. Although shorter lifetime of conventional columns becomes a problem under such a conditions, as Triart is based on an organic/inorganic hybrid particle, with an innovative surface modification, it offers excellent durability even under harsh eluent conditions such as 1% TFA and/or 70°C.
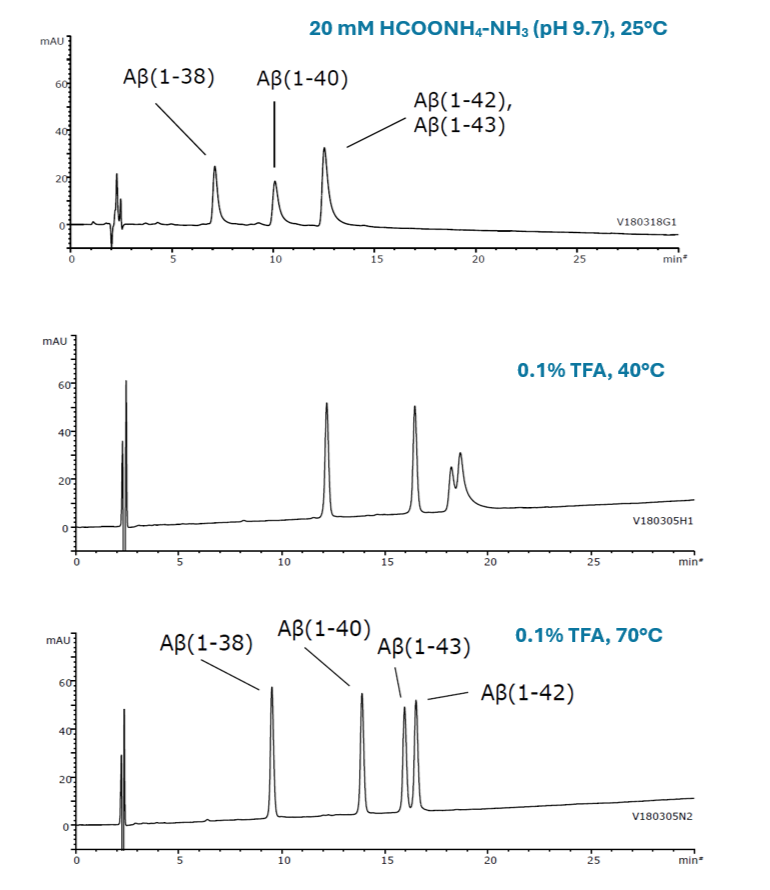

Amyloid β protein (Human)
Amyloid β (1-38) MW: 4,132
Amyloid β (1-40) MW: 4,330
Amyloid β (1-42) MW: 4,514
Amyloid β (1-43) MW: 4,615
Figure 16. Method optimisation of intact amyloid protein separation using both eluent pH (modifier) and temperature with the organic / inorganic hybrid YMC-Triart Bio C4
Conditions
| Columns |
YMC-Triart Bio C4 (5 μm, 300 Å), 150 x 3.0 mm ID
|
| Mobile phase |
A) water/buffer
B) acetonitrile/buffer |
| Flow rate | 0.4 mL/min |
| Gradient | 25-40%B (0-30 min), 90%B (30-40 min) |
| Detection | UV at 220 nm |
| Injection | 4 μL (each 0.1 mg/mL) |
Figure 16 shows the simultaneous analysis method of four Aβ proteins (1-38, 1-40, 1-43, 1-42) developed by changing pH (modifier) and temperature. The conditions containing 0.1% TFA at higher temperature provided better peak shape and resolution of Aβ(1-42) and Aβ(1-43).
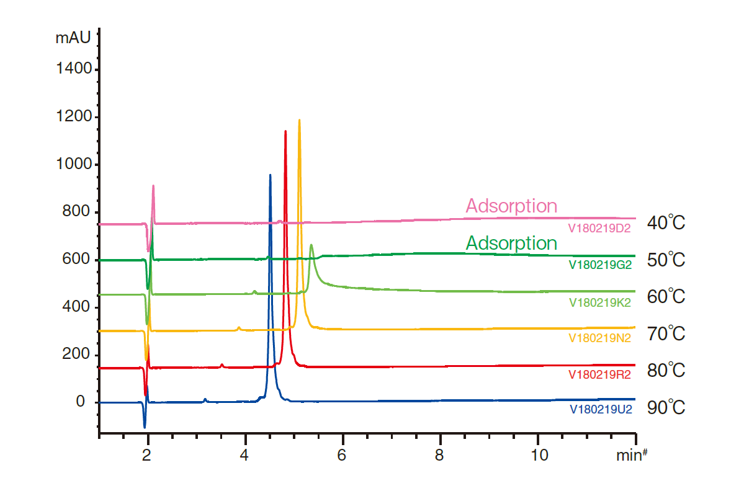

Figure 17. Peak shape improvement of intact Humanized monoclonal IgG1 with increasing temperature using the organic / inorganic hybrid YMC-Triart Bio C4
Conditions
| Columns |
YMC-Triart Bio C4 (3 µm, 300 Å), 150 X 3.0 mmI.D.
|
| Mobile phase |
A) water/TFA (100/0.1)
B) acetonitrile/TFA (100/0.1) |
| Flow rate | 0.4 mL/min |
| Gradient | 30-60%B (0-15 min), 90%B (15-30 min) |
| Detection | UV at 220 nm |
| Injection | 4 µL |
| Sample | Humanized monoclonal IgG1 |
Figure 17 shows the analysis of intact monoclonal antibody IgG1 at temperatures between 40°C and 90°C using Triart Bio C4. Good peak shapes were acquired at 70°C and higher while there was no elution at 50°C and lower. The highly durable Triart Bio C4 hybrid particle can perform stable analysis even at 90°C and is suitable for the reversed-phase analysis of antibodies at high temperatures.
Micro and Nanobore HPLC Columns
Often the amount of protein sample available for analysis is very lmited, and therefore in order to meet the sensitivity requirements for detection, the sample needs to undergo very little dilution on column which can be achieved using micro- or nano-bore columns, typically 1.0 - 1.5mm in diameter (microbore) or <<1mm (nanobore).
Protein standards are an important tool for testing LC-MS system suitability and intact protein mass measurement. Figure 18 shows the protein mix MSRT2 from Sigma- Aldrich was analysed using an YMC-Triart Bio C4 nano-bore (capillary) column (0.3mm i.d.).
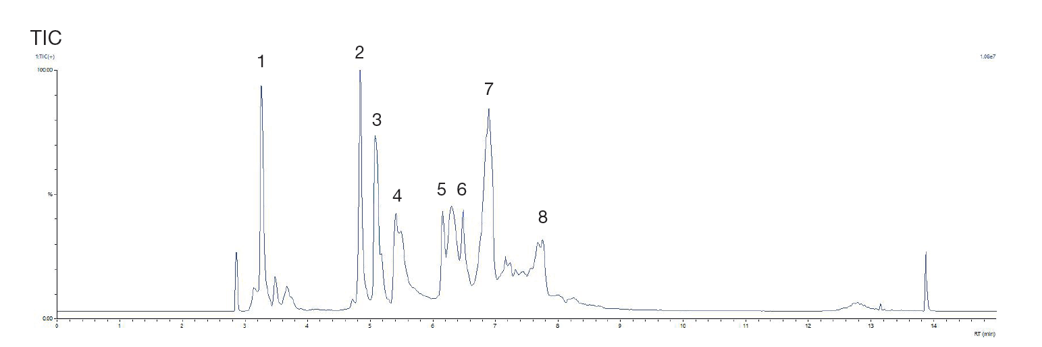

MSRT2 standard proteins detected;
- Ribonuclease B (1)
- Lysozyme (2)
- Insulin (3)
- Transferrin (4)
- Bovine serum albumin (5)
- Trypsin inhibitor (6)
- β-Lactoglubolin A (7)
- Lactate dehydrogenase (8)
Figure 18. TIC of the MSRT2 protein standard using YMC-Triart Bio C4 0.3mm i.d. column
Conditions
| Columns |
YMC-Triart Bio C4 (3 μm, 300A) 100 x 0.3 mm ID with 1/16" end fittings
|
| Mobile phase |
A) water + 0.1 % FA
B) acetonitrile + 0.1 % FA |
| Flow rate | 15 μL/min |
| Gradient | 20 % B (0–2.5 min), 20 %–60 % B (2.5–11 min), 60 %–100 % B (11–12 min), 100 % B (12–13 min), 20 % B (13–15 min) |
| Detection | Shimadzu LCMS-9030 QTOF |
| Injection | 0.1 μL |
| Sample | MSRT2 standard proteins 100 μg (Sigma-Aldrich), resuspended in 500 μL 0.1 % FA in water; concentration 0.2 μg/μL |
| Temperature | 50 °C |
Figure 18 shows good peak shape and excellent sensitivity using the YMC Triart Bio nanobore column, despite the low final concentration of the injected sample.
Figure 19 shows the separation of the analysis of proteins intact using a Thermo Scientific Accucore 150-C4 (150 Å pore diameter) nanoLC column (0.075mm). The analysis of five proteins (ranging in mass from 6 to 45 kDa) is carried out in 15 minutes with pressures compatible with conventional nanoLC instrumentation.


Peak Number Protein
1 Cytochrome C
2 Insulin
3 Myoglobin
4 Carbonic Anhydrase
5 Ovalbumin
Figure 19. UV chromatogram for five proteins separated on an Accucore 150-C4, 150 mm x 75 μm nano HPLC column (blank injection subtracted to compensate for the change in baseline
with acetonitrile concentration). * indicates an impurity from carbonic anhydrase.
Conditions
| Columns |
Accucore 150-C4, 2.6 µm, 150 x 75 µm
|
| Instrument |
Thermo Scientific EASY nLC II and Thermo Scientific Dionex UltiMate 3000 Rapid Separation Four Channel Variable Wavelength Detector (3 nL flow cell)
|
| Mobile phase |
A) 0.1 % formic acid in water
B) 0.1 % formic acid in acetonitrile |
| Flow rate | 300 nL/min |
| Gradient | 0 % B (0 min), 30 % B (1 min), 60 % B (11 min), 95 % B (12 min), 95 % B (15 min) |
| Injection | 0.25 µL 2 pmol/µL solution |
| Sample | A 1 mg/mL solution of each protein water - combined, with the resultant solution diluted to a final concentration of 2 pmol/μL. |
| Temperature | 40 °C |
| Backpressure (100% aqueous) | 204 bar |
| Run time | 15 minutes + equilibration time |
| UV detector wavelength | 214 nm |
Figure 19 shows all 5 proteins giving good peak shape with the 0.075mm i.d. columns, at pressures compatible with conventional nanoLC systems.
It should be noted that the use of nano-bore columns typically requires the use of specialist HPLC equipment, with very low extra column volume and pump systems capable of consistently delivering very low flow rates.
If micro- or nano-bore HPLC systems are not available, a newer approach uses an ‘HPLC optimised’ approach, in which 1.5mm i.d. columns are used with conventional HPLC or UHPLC systems which have been optimised to contain minimum extra column volumes. Figure 19 shows the chromatograms obtained for the intact trastuzumab protein using both 2.1 and 1.5 mm HALO 1000A Diphenyl columns.


Figure 20. UV of Intact trastuzumab on a 1.5mm ID column (Blue) and a 2.1mm ID column (red)
Conditions
| Columns | |
| Mobile phase |
A) Water/0.1% DFA
B) 50% Acetonitrile/50% n-propanol/0.1% DFA |
| Flow rate | 0.2 mL/min for 1.5 mm ID, 0.4 mL/min for 2.1 mm ID |
| Gradient | 27-36 % B in 40 min |
| Detection | PDA, 220 nm |
| Injection | 3 µL of 1.0 mg/mL Reduced and Alkylated Trastuzumab |
| Sample Solvent | Water/0.1% TFA |
| Temperature | 60 °C |
| Back Pressure | 252 bar (1.5 mm), 272 bar (2.1 mm) |
Trastuzumab was analysed in a top-down approach using the HALO® Diphenyl phase incorporating the new 1.5 mm ID column and compared to the 2.1 mm ID column. The 1.5 mm ID column showed enhanced sensitivity compared to the 2.1 mm ID, and provided increased solvent savings, reliability and robustness, making the 1.5 mm ID column a welcome addition to the HALO® family of products, and an ideal tool for proteomic analysis.
Summary
In this technical article, we have presented examples of intact protein analysis in reversed phase (denaturing) conditions.
We have seen that both the particle morphology, including particle size and pore size as well as the bonded phase can be optimised to ensure good protein retention, efficiency and selectivity. The nature of the pore size is dictated by the protein molecular weight and hydrodynamic volume and pore size needs to be carefully considered for successful protein retention. We have seen the importance of using various stationary phase chemistries to ensure selectivity is fully explored and important protein variants are not missed.
We have also considered the importance of flexibility in both eluent pH (modifier used) and temperature and the clear advantages that these variables can deliver in optimising intact protein separations.
The use of micro- and nano-bore columns has also been explored as an ideal solution when sample volumes are limited.
With our wide range of columns from world class manufacturers, back by excellent technical support, Element Lab Solutions are your ideal partner for intact protein analysis.