Gas chromatography (GC) is a critical analytical technique in various scientific fields, and the choice of mobile phase (or carrier gas) plays a significant role in its efficiency and effectiveness. Traditionally, helium has been the preferred carrier gas choice, but the benefits of switching to hydrogen are increasingly recognized. This article explores the advantages of using hydrogen over helium in GC.
Helium has been the most popular carrier gas for GC due to its chemical inertness and the ability to provide faster separations in comparison to nitrogen, and traditionally was thought to be safer than hydrogen gas [1]. However, helium, although abundant in the universe, is a scarce resource here on Earth and is predominantly produced via fractional distillation of natural gas. During the past decade, this scarcity has led to several helium shortages and steep increases in cost making helium a less attractive option for many laboratories [2]. Although it is not just the lack of resources and the high cost which needs to be considered, the environmental impact helium production has, as a byproduct of fossil fuel production, helium is gaining a reputation as the least sustainable carrier gas choice for GC.
However, there is a way to produce fast, high efficiency, separations while being more environmentally conscious and that’s by making the switch to hydrogen as a carrier gas.
Hydrogen
In principle, hydrogen carrier gas is suitable for almost all GC methods, except of course for the analysis of hydrogen as a component in a sample mixture. The GC method must be converted to conditions suitable for hydrogen, and detectors may require special consideration.
Flame ionization detector (FID) also uses hydrogen as a support gas; total FID hydrogen flow should be kept constant and stoichiometric ratios optimised according to the manufacturer’s recommendations. Some electron capture detectors (ECD) support hydrogen carrier gas while others may not; it is best to consult the manufacturer in each case. Thermal conductivity detectors (TCD) will function well, but absolute response (as opposed to sensitivity) may not be the same as with helium carrier.
When using GC-FID the switch from helium to hydrogen can be a relatively straight forward process as FID is very well suited to use with hydrogen. Many applications can be transferred and optimized using in-silico method translation software to predict how chromatograms might change and the optimum conditions and instrument parameters to achieve the ideal chromatography. Figure 1 shows how small changes to a method can result in reduced run times, improved peak shape and reduced peak widths using hydrogen over helium carrier gas:


Figure 1. Agilent technologies in-silico method translation tool…comparison GC-FID chromatograms showing a residual solvents method ran using helium and an optimised hydrogen carrier gas method [3]
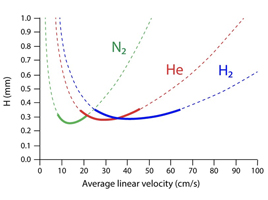
While helium and hydrogen are both very efficient gases for GC use and can be switched from one to the other relatively easily, ensuring little differences in separation quality, the difference in properties allows hydrogen to produce a more efficient separation [2]. At the same temperature and pressure hydrogen is half as viscous as helium, therefore hydrogen travels through a GC column faster resulting in a quicker analysis time of the same samples [3]. Additionally, hydrogen diffusivity is higher compared to helium thus allowing the use of higher flow rates, which can also lead to a reduction in retention times, further increasing the speed of analysis. Figure 2 shows that the Van Deemter minimum (highest efficiency) for hydrogen carrier gas occurs at higher linear velocity than either helium or nitrogen.


Figure 2: van Deemter curve showing the relative efficiencies of hydrogen, helium, and nitrogen at different flow rates [4]


Figure 3: Relative viscosity of common GC carrier gases at various temperatures [5]
One of the main issues for laboratories to make the switch from helium to hydrogen is the safety concerns of using hydrogen gas within the laboratory. Traditionally gas is supplied via the use of cylinders, which have the potential to release vast quantities of gas into the lab, whereas the use of a generator eliminates this potential problem [6].
Hydrogen generators
Utilizing hydrogen generators over helium cylinders offers a wide range of advantages, including cost savings, safety benefits and a reduction in environmental impact.
Hydrogen generators offer a safer alternative to cylinders, meeting diverse pressure and flow needs. They produce hydrogen through water electrolysis, often using a proton exchange membrane (PEM) to separate hydrogen from byproducts. Generators reduce manual handling risks since they only require purified water, and hydrogen is produced on-demand, minimizing stored gas volume
Many hydrogen generators have the following built in safety features or these are features which should be considered when selecting a generator provider.
- Forced air ventilation within the generator housing to minimise accumulation of hydrogen/air mixtures
- Self-tests to check for leaks on power up or initiating hydrogen production mode following long periods of inactivity, which shut off hydrogen production if a leak is detected
- Sensing of pressure / flow setpoint achievement and automated shut down if these cannot be achieved
- Pressure sensing within the unit which will shut down the generator if an over-pressure is sensed – shut down typically takes the form of interrupting the current to the cell so that gas production ceases, and a pressure relief valve is opened to reduce pressure within the hydrogen storage reservoir
- Sensors to detector when the water reservoir is running low
- Hydrogen detection feedback which senses a gas leak within the instrument or within the laboratory environment and shuts down hydrogen production within the generator
One of the leading manufacturers of hydrogen generators is PEAK Scientific, a one-time purchase of a hydrogen generator can lead to a reduction in carbon footprint as the need for continuous deliveries is alleviated. Furthermore, the environmental impact of hydrogen is reduced by using generators in comparison to helium production, as the generation is 100% renewable from de-ionized water.
Sustainability is a growing concern for many laboratories and switching from helium to hydrogen can help improve green credentials as labs requiring helium gas for their GC instrument to meet 1LPM (liter per minute) demand for a lab operating 12 hours per day, 23 days per month can save on 24 cylinders requiring delivery per year with a hydrogen generator. Additionally, helium production on average produces 500g CO2/L He whereas using a PEAK Scientific Trace Hydrogen generator which uses 0.787 kWh at full flow 0.5L/min could result in significant CO2 reductions, e.g.:
Generator: 0.5L x 60 min = 30L per hour @ 0.787 kWh = 0.168kgCO2 (12 hours @ 30L/h = 2 kgCO2) compared to helium (500g x 360L) = 180kgCO2 produced for the same volume of gas required. This highlights the potential CO2 emission reduction when switching to a Hydrogen generator on a laboratory scale, however this does not consider any transportation or manufacturing emissions, although as previously stated with generators it is a one-time delivery compared to repeated cylinder deliveries which will all affect the total CO2 emissions related to each option.
Hydrogen and Mass Spectrometry
Using hydrogen with GCMS systems presents some further issues for consideration. Hydrogen typically has higher linear velocity (or flow rate per column diameter) to take advantage of its
inherently higher chromatographic efficiency and lower compressibility. It is therefore recommended that higher-capacity vacuum pumps be used to avoid hydrogen accumulation in the spectrometer, as its higher diffusivity and lower viscosity make it inherently more difficult to pump away than helium or nitrogen. This is particularly important as the filaments used in the mass spectrometer ion source operate at extremely high temperatures and can be an ignition source. Some manufacturers offer an inert gas venting system, such that if the vacuum system should fail, the carrier gas is immediately flushed from within the ion source and mass analyser using a separate flow of nitrogen or other inert flush gas.
Other considerations for using hydrogen with mass spectrometry include optimising the source components and noting that hydrogen might alter the metallurgic properties of some metals. Three components which need to be optimised are:
- The magnet to ensure it is hydrogen compatible.
- The draw out lens might need altering for a hydrogen draw out lens (typically with a different orifice diameter) to ensure good sensitivity
- The ion source body and materials of construction
Agilent have released a Hydro inert ion source which is designed to reduce sensitivity loss, improve peak shape and retain spectral fidelity while also reducing maintenance and ion source cleaning. Another consideration is the need for conditioning as hydrogen is more likely than helium to displace contaminants from roughened or upswept surfaces within the system, conditioning protocols often include running at the highest source temperatures, reduced detector (electron multiplier) voltage with the filament turned on, overnight.
The chromatograms below demonstrate the importance of optimizing the mass spectrometer ion source components and conditioning to improve peak shape and reduce chromatographic and spectral background.


Figure 4. Mass spectra before and after optimisation and conditioning protocol: Use 6 mm draw out lens, set the source to max temperature, reduce the EMV to <= 800V and leave the FILAMENT ON overnight. Column: Agilent J&W DB-5MS UI 20m x 0.18mm id x 0.18 um.
Summary
This article explores the transition from helium to hydrogen as a carrier gas in gas chromatography (GC), emphasizing its economic, environmental, and operational benefits. Helium, traditionally preferred for its inertness and efficiency, has become less attractive due to its rising costs, scarcity, and environmental impact. Hydrogen offers inherently faster separations and reduced retention times due to its lower viscosity and higher diffusivity. Laboratories can mitigate safety concerns with hydrogen by using generators, which produce gas on demand, reducing the risks associated with stored gas. Generators also feature advanced safety mechanisms, such as leak detection and pressure monitoring.
Switching to hydrogen supports sustainability goals, significantly cutting carbon emissions. For example, a reduced CO₂ output by up to 178 kg could be achieved when replacing helium cylinders with a hydrogen generator for a typical laboratory analysis. Additional savings stem from reduced transportation emissions and lower energy use in hydrogen production. Advanced solutions like Agilent’s Hydro Inert Ion Source enhance GC-MS performance with hydrogen carrier gas, ensuring compatibility, improved sensitivity, and easier maintenance. Overall, hydrogen presents a cost-effective, safer, and more environmentally conscious alternative for GC applications.
Contact us to talk to an Element Laboratory Solutions sales representative to discuss switching from Helium to Hydrogen and gas generator options.
Resources
[1] Restek. (2013). Benefits and Considerations of Converting to Hydrogen Carrier Gas. [online] Available at: https://www.restek.com/global/en/articles/benefits-and-considerations-of-converting-to-hydrogen-carrier-gas [30th May 2024].
[2] LCGC North America. (2010). Considerations for Switching from Helium to Hydrogen. [online] Available at: https://www.chromatographyonline.com/view/considerations-switching-helium-hydrogen [Accessed 30th May 2024]. [3] Agilent Technologies, CA, Residual Solvents Analysis for the Pharmaceutical Industry Using the Agilent 8697 Headspace Sampler and 8850 GC-FID System
[4] Peak Scientific. (2018). The Effect of Draw Out Lens Diameter on Sensitivity of GC. [online] Available at: https://www.peakscientific.com/discover/articles/the-effect-of-draw-out-lens-diameter-on-sensitivity-of-gc/ [Accessed 10 Sep. 2024].
[5] J.V. Hinshaw, Column Connections, LCGC Asia Pacific, 12(2), 1100 (2009)
[6] PEAK Scientific. (2017). The benefits of gas generators over gas cylinders: Expert opinion. [online] Available at: https://www.peakscientific.com/discover/articles/benefits-of-gas-generators-over-gas-cylinders-expert-opinion/ [Accessed 12 Oct. 2024].