GLP-1 drugs, or glucagon-like peptide-1 receptor agonists, are a class of medications used primarily to treat type 2 diabetes and obesity. They mimic the action of a naturally occurring GLP-1 hormone which helps regulate blood sugar and appetite. These drugs have proven extremely effective for weight loss, including for people without diabetes.
GLP-1 drugs work in several ways including stimulating insulin release when sugar levels are high, supressing glucagon, slowing down stomach emptying and reducing appetite.
There are several GLP-1 drugs currently marketed including Semaglutide (brand names: Ozempic, Wegovy, Rybelsus), Tirzepatide (Mounjaro, Zepbound), Liraglutide (Victoza, Saxenda), Dulaglutide (Trulicity) and Exenatide (Byetta, Bydureon).
HPLC is a critical analytical tool in GLP-1 development, production and quality control.
Purity and Impurity Profiling
- HPLC helps detect and quantify impurities, degradation products, post-translational modifications (PTM’s) and by-products in the final drug formulation.
- Regulatory bodies like the FDA and EMA require stringent impurity profiling, and HPLC is usually the primary tool for that.
Peptide Mapping
- In GLP-1 analogs (e.g., semaglutide, liraglutide), peptide mapping is used to confirm the sequence, modifications, and structure, for example a fatty acid side chain modification as well as amino acid sequence differences of the GLP-1 peptide chain in the case of Semaglutide (Figure 1)
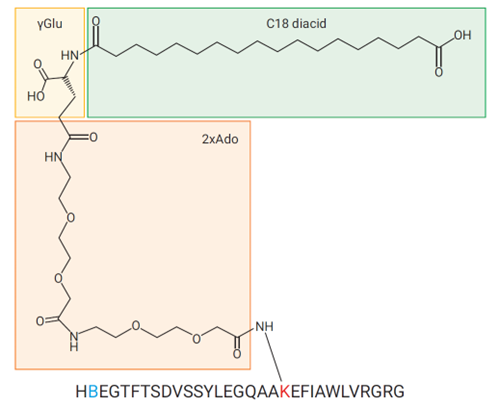

Figure 1: Structure of semaglutide showing differences to a native GLP-1 fragment sequence.
- This ensures that the molecule being produced is exactly what it's intended to be - especially important for biosimilars and synthetic peptide drugs.
Stability Testing
- HPLC is used to test how stable a GLP-1 drug is over time under different storage conditions.
- It identifies chemical degradation, oxidation, deamidation, and aggregation.
Pharmacokinetic Studies
- In preclinical and clinical trials, HPLC is used (often coupled with mass spectrometry) to quantify how much of the drug is present in plasma or serum samples over time.
Formulation Development
- Different formulations (injection, oral) require stability and compatibility testing.
- HPLC ensures the active pharmaceutical ingredient (API) remains stable in various excipients or delivery systems.
HPLC analysis of peptides presents several challenges including peptide instability, non-specific binding and the complexity of the peptide mixture. Most peptide drugs are produced using solid-phase support or resin, which can easily be filtered from reactions. Multiple deprotection, activation and coupling steps are involved in the synthesis resulting in impurities and scavenger products being abundant in the final peptide mixture, often leading to a requirement for purification.
Reversed-phase HPLC using an acetonitrile gradient with an ion pair (typically 0.1% TFA) is used for the analysis. Generally structural confirmation requires mass spectrometric detection. This presents a problem, since while 0.1% TFA is required for superior peak shape and optimum retention, it also causes ion suppression, resulting in a weaker MS signal. As a compromise, formic acid (FA) is generally used for LC/MS methods, this has a weaker ion pairing effect than TFA and therefore doesn’t compromise the MS signal.
However, FA is less effective at suppressing nonspecific interactions and creates a less hydrophobic (and therefore less retentive) ion pair with the peptide. Consequently, resolution when using FA as an ion-pair reagent can be compromised. Choosing different column chemistries can greatly enhance separation selectivity for certain impurities, increasing the confidence in the ability to detect and quantify modifications present in the sample.
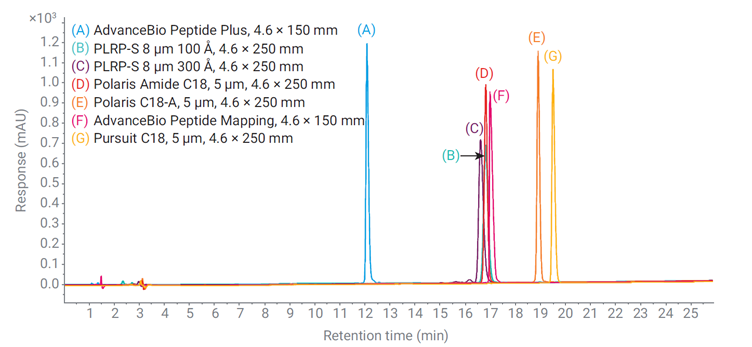



Figure 2: Comparison of analytical LC-UV chromatograms of semaglutide under 0.1% TFA conditions (Top) and 0.1% FA (Bottom) using optimised gradient (see conditions below).
| Mobile Phase | Eluent A1: 0.1% TFA in Water Eluent B1: 0.1% TFA in ACN Eluent A2: 0.1% FA in Water Eluent B2: 0.1% FA in ACN |
| Flow Rate | 1.0 mL/min |
| Gradient | 35 to 60% B in 30 mins (Top Figure, TFA Ion Pair Reagent) 30 to 55% B in 30 mins (Bottom Figure, FA Ion Pair Reagent) |
| Column Temperature | 25 °C |
| Injection Volume | 10 μL |
| Detection | UV, 220 nm |
| Total Run Time | 30 minutes |
Figure 2 (Top) shows overlaid chromatograms from a single injection of semaglutide reference material across seven different columns from Agilent Technologies. Two of these columns, packed with superficially porous particles, were 150 mm long, while the rest were 250 mm long. Despite these differences, all columns produced sharp, symmetrical peaks. The polystyrene/divinylbenzene columns showed broader peaks, consistent with their larger particle size. The AdvanceBio Peptide Plus column exhibited a shorter retention time due to its lower bonding density which results from a charged surface modification. The Polaris C18-A and Pursuit C18 columns showed the longest retention times, likely reflecting their higher surface areas.
Using formic acid (FA) instead of trifluoroacetic acid (TFA) as the ion-pairing reagent required gradient adjustment due to FA’s weaker acidity and lower hydrophobicity. Figure 2 (Bottom) shows the resulting chromatograms, revealing generally poorer peak shapes on some columns. This was attributed to unwanted interactions between peptide basic residues and acidic silanol groups on the silica surface. However, the AdvanceBio Peptide Plus, Polaris Amide C18, and Polaris C18-A columns maintained good peak shapes. Their superior performance is likely due to bonding chemistries with residual positive charges that minimise secondary interactions and peak tailing.


Figure 3: Comparison of heat-treated semaglutide LC-UV chromatograms showing the effect of ion‑pair reagents TFA (A) using grdaients shown in the conditions previously discussed, with the Agilent AdvanceBio Peptide Plus column.
Liraglutide is a full agonist of the GLP-1 receptor and shares 97% of its amino acid sequence identity with human GLP-1. It was created by substituting arginine for lysine at position 34 in the GLP‐1 peptide and adding a palmitic acid chain with a glutamic acid spacer on the lysine residue at position 26 to improve the pharmacokinetic effects (Figure 4).


Figure 4: Structure of liraglutide
High resolution accurate mass (HRAM) mass spectrometry can be used to identify and quantify various Liraglutide impurities, which is vital to assessing the critical quality attributes of commercial synthetic peptides. However, the process of identifying and quantifying impurities can be made much simpler when a good chromatographic separation of the analytes can be achieved.


Figure 5: The zoomed TIC of a liraglutide sample and the separation of the main peak and impurities
| Column | Agilent AdvanceBio Peptide Plus, 2.1 × 150 mm, 2.7 μm |
| Mobile Phase | 0.01% TFA in 95:5 Milli-Q Water: Acetonitrile 0.01% TFA in 95:5 Acetonitrile:Milli-Q water |
| Flow Rate | 0.4 mL/min |
| Gradient | 0 to 3 mins. 10% B 3 to 6 mins. 10% - 40% B 6 to 50 mins. 40% - 50% B 50 to 55 mins. 50 to 65% B |
| Column Temperature | 45 °C |
| Injection Volume | 1 μL |
| Detection | UV, 220 nm |
| Total Run Time | 60 minutes |
The Agilent Bioconfirm LC/MS approach for a therapeutic peptide workflow uses the chromatographic separation, in-spectrum dynamic range, isotopic fidelity, and accurate mass for identification and impurity profiling. The Agilent AdvanceBio Peptide Plus stationary phase uses chemically modified silica with a charged hybrid C18 surface to offer alternate selectivity over traditional C18 columns, resulting in improved separation of liraglutide impurities. The total ion chromatogram (TIC) (Figure 5) shows that minor peaks eluting before and after the main peak are also observed.
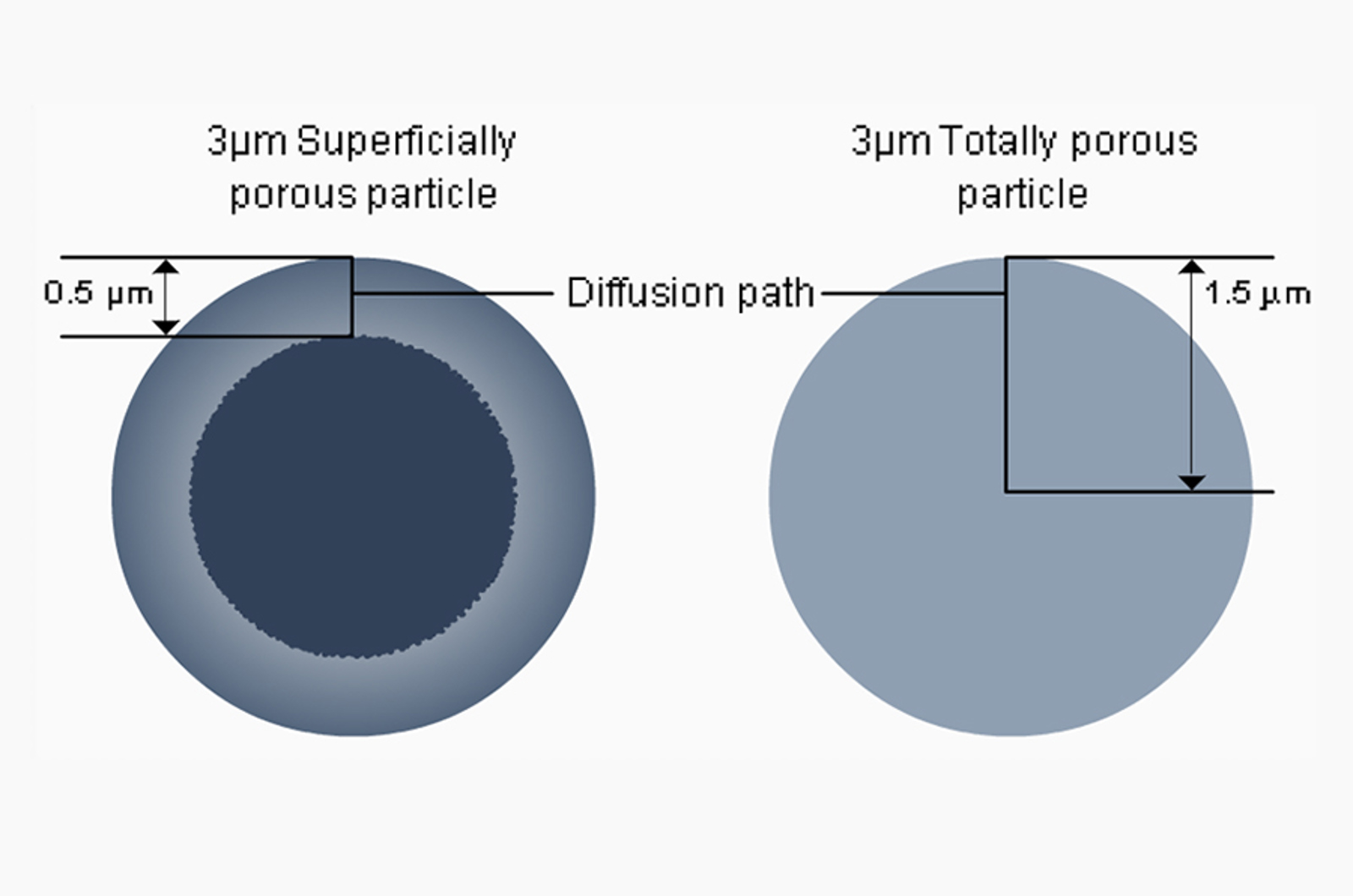
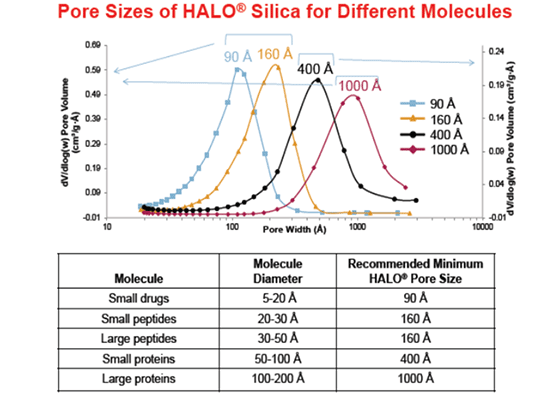
A further consideration in HPLC analysis is the size of the peptide, since choosing the correct particle pore size is important for ensuring interaction with the bonded phase and optimising peak shape. Although smaller than proteins, which generally require a 300 Å pore size particle, peptides are bigger than small molecule drugs. GLP-1 drugs are generally in the range 3000-5000 Daltons, indicating the requirement of an intermediate pore size of 160-200 Å for the analysis. This being said, the analysis of GLP1 type peptides using 300 Å pore size media may also be appropriate depending upon the size (hydrodynamic volume) of the peptide in solution.


Figure 6: LC-UV chromatogram comparison of heat-treated semaglutide using Agilent PLRP-S columns, 100Å (A) and 300Å (B).
This is best illustrated in Figure 6, which shows the same degraded sample analysed on the polymeric PLRP-S 100 Å column versus PLRP-S 300 Å column. The resolution was clearly improved on the 300 Å column and demonstrates the way in which larger pores do not restrict the mass transfer in and out of the stationary phase to the same extent as smaller pore sized columns, and hence sharper peaks may be obtained.
An important aspect of any peptide analytical strategy is the ability to scale to semi-preparative and preparative chromatography. The GLP-1 lipopeptide is challenging to achieve efficient purification strategies, and for the purification of GLP-1, alkaline buffers are very beneficial for both effective binding and separation. However, silica based stationary phases are unstable at high pH. YMC-Triart Prep is based on a hybrid organic / silica chemistry and due to ethylene bridge cross-linking, it provides exceptional chemical stability allowing it to be used at high pH. This is advantageous for many peptide purifications and drastically increases the possible method options. Furthermore YMC-Triart Prep stationary phases enable reproducible results when using 100% aqueous conditions which is particularly useful for polar peptide isolation.
Figure 7 shows a two-stage purification of Tirzepatide, which highlights the utility of the Triart preparative materials which isolates a 99% pure product from a 20% pure crude feedstock.


Figure 7: Two stage preparative purification of Tirzepatide using YMC-Triart Prep Bio200 C8 (first stage) and YMC-Triart Prep C4-S (second, polishing, stage).
The method development was performed at analytical scale screening the complete YMC-Triart Prep set-up. The best results regarding separation efficiency, target purity and recovery rate were obtained with YMC-Triart Prep Bio200 C8, a C8-modified stationary phase with lower hydrophobicity and optimised pore size (200Å) for crude peptide purifications. During method development the chromatographic parameters including mobile phase composition and organic modifier were adapted to improve the overall separation results. Subsequently, the loading was increased to improve the productivity of the purification.
First stage preparative conditions;
| Column | YMC-Triart Prep Bio200 C8 (10 μm) 250 x 10 mm ID |
| Eluent | A) 20 mM CH3COONH4 B) Acetonitrile |
| Gradient | 33.5%B (0-5 min) 33.5-41%B (5–55 min) |
| Flow rate | 2.4 mL/min |
| Temperature | 25 °C |
| Detection | UV at 280 nm |
| Injection | 4 mL |
| Sample | 20 mg/mL Tirzepatide crude in 20 mM NH4HCO3 (80/20) |
The second chromatography step in this purification process, which was necessary to achieve the required purity of >99.5%, was a polishing step via RP. This step was carried out with YMC-Triart Prep C4-S which gave the best results in the stationary phase screening in combination with adjusting the method parameters. The purification was performed at pH 8.0 leading to highly efficient separation results. This is possible due to the innovative hybrid-silica base of the YMC-Triart Prep stationary phases and combined with the less hydrophobic C4 modification this offers an ideal modification for a polishing purification of Tirzepatide and other challenging GLP-1 peptides.
Polishing stage preparative conditions;
| Column | YMC-Triart Prep C4-S (10 μm) 150 x 4.6 mm ID |
| Eluent | A) 50 mM Ammonium Hydrogen Carbonate B) Acetonitrile |
| Gradient | 31%B (0–5 min) 31-35.5%B (5–35 min) |
| Flow rate | 0.5 mL/min |
| Temperature | 25 °C |
| Detection | UV at 280 nm |
| Injection | 150 μL (3 mg load) |
| Sample | Tirzepatide crude after purification step 1, 20 mg/mL in 20 mM NH4HCO3/Acetonitrile (80/20) |
A wide range of pre-packed columns and loose material are available for self-packing to allow a high degree of flexibility at both semi-preparative and preparative scale using the Triart Prep range of phases. Many of the pre-packed columns are available in Accura hardware, which is deactivated, to produce the lowest peptide adsorption and the most symmetrical and efficient peaks possible.
Beside the purification process development, the process control can also be achieved with the highly stable YMC-Triart series. Figure 8 shows the analytical control separation using YMC-Triart Bio C18 (1.9 μm) in analytical dimensions used to successfully monitor the purity level of the Tirzepatide crude and purification fractions.


Figure 8: YMC-Triart Bio C18 stationary phase used for the process control of Tirzepatide purification.
| Column | YMC-Triart Bio C18 (1.9 μm) 100 × 2.1 mm ID |
| Eluent | A) 20 mM Ammonium Formate/Acetonitrile (95/5) B) 20 mM Ammonium Formate /acetonitrile (5/95) |
| Gradient | 34.4-43.3%B (0–16 min) 100%B (16-20 min) |
| Flow rate | 0.20 mL/min |
| Temperature | 60 °C |
| Detection | UV at 220 nm |
Phases in the YMC Triart Prep series are available in analytical dimensions and several particle sizes, including UHPLC (sub 2mm) compatible particle diameter.
As discussed above, higher pH is often required for the efficient preparative isolation of lipopeptides such as the GLP-1 derivatives and Figure 9 shows the successful single stage isolation of Liraglutide (antidiabetic peptide therapeutic, market by Novo Nordisk as Victoza®). This method uses the YMC-Triart Prep C18-S, which is stable in the pH range 2-10, under alkaline conditions to achieve a single stage purity of >99.5%. This phase is well suited to simple scaling from analytical, through preparative to manufacturing scale.


Figure 9: YMC-Triart Prep C18-S stationary phase used for the single stage purification of
| Column | YMC-Triart Bio C18 (1.9 μm) 100 × 2.1 mm ID |
| Eluent | A) 20 mM Ammonium Formate/Acetonitrile (95/5) B) 20 mM Ammonium Formate/Acetonitrile (5/95) |
| Gradient | 34.4-43.3%B (0–16 min 100%B (16-20 min) |
| Flow rate | 0.20 mL/min |
| Temperature | 60 °C |
| Detection | UV at 220 nm |
Common cleaning-in-place (CIP) procedures with 0.1 M NaOH can be used to regenerate the column and restore chromatographic performance. The YMC-Triart Prep stationary phases are stable for more than 300 CIP cycles and figure 10 shows a performance comparison of YMC-Triart Prep media using a high pH cleaning in place protocol (below) against a competitor media.




Figure 10: YMC-Triart Prep Bio200 C8 stationary phase performing well after 300 cleaning in place (CIP) cycles as opposed to a competitor product which shows significant peak deterioration after 95 cycles .
With its robustness and long lifetime, this packing media reduces process costs enormously. In combination with the excellent chromatographic performance and high loadability, YMC-Triart Prep can contribute to higher productivity of the overall process. Further the ability to implement cleaning in place protocols with extremes of pH without significant performance deterioration reduces production costs due to lower consumption of packing material and less downtime due to column repacking.
Conclusion
This technical note has presented several solutions for the analysis and preparative isolation of GLP-1 drugs, a class of medications used primarily to treat type 2 diabetes and obesity, whose popularity has grown exponentially in recent years. We have demonstrated that the charged surface of the Agilent AdvanceBio Peptide Plus stationary phase is ideal for purity measurement and impurity profiling, even when using formic acid as the ion-pair reagent to allow the use of mass spectral detection.
We have also highlighted the utility of the AdvanceBio Peptide Plus phase in producing excellent selectivity and high resolution in the MS characterisation of GLP-1 peptide characterisation studies.
We have shown the importance of selecting the correct stationary phase pore size when using both silica and polymer based stationary phase support materials and the effect of pore size on the mass transfer kinetics, and hence peak width, when undertaking peptide impurity studies.
Preparative separations using YMC Triart Prep phases have been shown, which highlight the selectivity range of these phases and their use in efficiently optimising the recovery of high purity product in the lowest elution volumes. These materials are also resistant to extremes of pH which enables effective cleaning in place regimes to be implemented, offering significant improvements in cost and efficiency of producing GLP-1 peptides at scale.