As HPLC methods evolve to meet the increasing demands of sensitivity, reproducibility, and precision, the importance of hardware quality and surface chemistry becomes more pronounced. One critical innovation that addresses these concerns is the use of surface passivated hardware, often known as Bio Inert hardware.
Traditionally, HPLC columns have been made from 316L or 316 stainless-steel. This material offers several advantages for HPLC, being able to withstand high operating pressure often up to 6000psi (or higher for UHPLC!), having excellent mechanical stability and good chemical resistance to typical mobile phases.
However, the stainless steel used in HPLC column bodies and frits (porous, typically sintered disks at either end of the column and contained with the column end-fittings) used to retain the packing material is prone to detrimental interactions with certain analyte types [1 – 5]. This results in lower sensitivity, peak broadening and peak asymmetry as analytes interact with neutral or charged metal ions (including those contained within surface metal-oxide layers) which can act as Lewis Acids (i.e. can accept donor electrons). Analytes containing phosphate or carboxylate groups are susceptible to adsorption to the metal surface and the effects are exacerbated as the number of these groups within the analyte molecule increase.
Using the Lewis acid definition of the surface metal ions, any species that can act as a Lewis Base (i.e. can donate non-bonding electrons) can potentially adsorb to these sites, forming non-covalent complexes. Analytes such as biomolecules, proteins, glycans, peptides, pesticides, phosphorylated or phosphonated species, anionic metabolites and amino acids are known to potentially suffer from adsorption to the HPLC frit and column body. The nature of interactions with the metal surface can include complexation, oxidation and epimerization reactions. This effect can be exacerbated when using eluent systems at acidic pH where it is likely surface metal ions will be positively charged and undergo ionic interactions with any negatively charged analytes (Figure 1);


Figure 1: Schematic showing the effects of metal surface coating in combating the unwanted secondary interactions between a phosphorylated anionic analyte and the cationic metal surface within an HPLC column or system
It is also well characterised that over time, especially when exposed to acidic or halide containing mobile phases, stainless steel can corrode, leaking iron (Fe) ions into the mobile phase and settling onto the silica packing material, leading to further reactivity and peak shape issues [6].
It should also be noted that metal ions in solution or deposited onto silica or metal surfaces are able to catalyse unwanted on-column reactions (degradation), including, for example, oxidation, amination, nitrosylation, or nitrosation.
Several approaches have been used to mitigate these unwanted interactions and reactions, these are summarised below, alongside some of their potential downsides or fallibilities;
Chemical deactivation (in-situ) – the use of various acidic species and chelators, including phorphoric acid, citric acid, etidronic acid, medronic acid, nitric acid or ethylenediaminetetraacetic acid (EDTA) have been used to mitigate secondary mechanisms of analyte binding. However, all these approaches have associated disadvantages, including the reversibility of chemical deactivation when carried out off-line, i.e. the columns are pre-treated and the reagents not added to the mobile phase. Chelators such as citric acid, acetylacetone, and medronic acid can cause ion-suppression and tend to linger in the system, especially in the ion-source when mass spectrometric detection is used. [7-9]
PEEK Lined Columns – the plastic, polyether ether ketone (PEEK), has been employed to avoid chelation / adsorption effects, but suffers from limited pressure resistance and some solvent incompatibilities, especially tetrahydrofuran. PEEK is also relatively hydrophobic and may require conditioning to avoid losses of hydrophobic analytes [10]
Alternative materials of construction – titanium and the nickel-cobalt alloy MP35N have been used for column and frit construction due to their improved corrosion resistance, however titanium can pit or crack under prolonged exposure to methanol and can also leach titanium ions into the packing material, leading to peak shape deformation [11]. Under certain conditions, MP35N can also leach cobalt and nickel ions into the silica bed causing activation.
More recently, column manufacturers have turned to Chemical Vapour Deposition (CVD) of a range of metal coating materials which can provide a more permanent and higher performance deactivation of stainless steel or titanium and these include the use of Dursan® (a registered trademark of SilcoTek Corporation), which is a specially functionalised amorphous silica oxide (a-SiO) as well as hybrid organic-inorganic compounds using ethylene-bridged siloxane polymers (known also as C2 or C2C10 coatings), which can be then further functionalised (in-situ) with a range ‘end-capping’ chemistries such as phenyl or diol [12, 13].
The nature of the ‘inert’ coating material and the degree and type of functionalisation vary from manufacturer to manufacturer as they seek the ideal coating to prevent peak shape deformation or unwanted analyte reactions (decomposition). As a further insight, the Dursan coating process is described as the decomposition of an alkylsilane followed by an oxidative exposure of the base material. The surface is then functionalized with a second alkylsilane to give a wear-resistant, hydrophobic and chemically inert coating [14, 15].
CVD is a non-line of sight technique in which the deposition of the coating material is achieved in the gas phase, and can therefore be used to achieve very effective surface coverage, which is especially important when considering the tortuous surface characteristics of a sintered stainless steel or titanium frit, for example. The CVD technique has been used to provide inertness to HPLC frits and column bodies as well as various components within the fluid path of HPLC systems.
Several manufacturers have employed these inert coatings to improve the chromatographic performance of a wide range of applications including small molecules, peptides, proteins, oligonucleotides and more. The range of column types and dimensions available with ‘inert’ hardware continues to grow and we highlight below some key applications in which the advantages of deactivated hardware are particularly useful.
YMC Triart is an organic-inorganic hybrid stationary phase particle with exceptionally narrow particle size distribution and triple end-capping to provide superior chromatographic performance and is available in 1.9, 3 and 5mm particle sizes. It is pH stable It is also available as Accura Triart, which has a bioinert coating on all of the column metal parts including the column body and frits. YMC report that their bioinert coating is hundreds of times thicker than offered by other manufacturers, making their column hardware coating highly inert and more durable.
Phosphate nucleosides are known to be highly coordinating compounds and are highly sensitive to activated metal sites within HPLC columns, undergoing strong secondary interactions via the phosphate groups, leading to broad and tailing peaks. These compounds are often used as probes to evaluate the quality of metal surface coatings and Figure 2 shows the difference in chromatography:
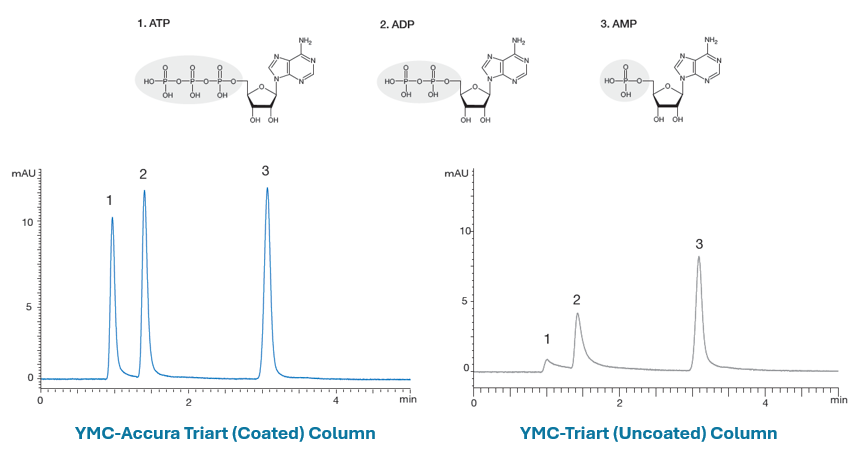

Figure 2: Improved sensitivity and peak shape for phosphate nucleotide coordination compounds obtained from YMC-Triart (Accura) coated columns versus the same stationary phase packed in uncoated stainless steel hardware
Figure 2 Conditions;
| Column: |
YMC-Accura Triart C18 (1.9 μm, 120Å) 50 x 2.1 mm ID (bioinert hardware) YMC-Triart C18 (1.9 μm, 120 Å) 50 x 2.1 mm ID (standard hardware) |
| Eluent: | 5 mM Ammonium Formate |
| Flow Rate: | 0.21 mL/min |
| Temperature: | 25 °C |
| Detection: | UV at 265 nm |
| Injection: | 1 μL (10 μg/mL) |
| System: | bioinert HPLC system |
Resistance to secondary interactions containing phosphate groups is particularly useful when analysing phosphorothioated oligonucleotides and Figure 3 shows the improvement in peak shape and peak height obtained when an identical stationary phase is used in coated hardware versus uncoated for the analysis of phosphorothioated oligonucleotides.


Figure 3: Improved sensitivity for phosphorothioated oligonucleotides obtained from YMC-Triart (Accura) coated columns versus the same stationary phase packed in uncoated stainless steel hardware
Figure 3 Conditions;
| Column: |
YMC-Accura Triart C18 (1.9 μm, 120Å) 50 x 2.1 mm ID (bioinert hardware) YMC-Triart C18 (1.9 μm, 120 Å) 50 x 2.1 mm ID (standard hardware) |
| Eluent: |
A) 15 mM triethylamine - 400 mM HFIP* |
| Gradient: | 8–18%B (0–10 min) |
| Flow Rate: | 0.42 mL/min |
| Temperature: | 65 °C |
| Detection: | UV at 260 nm |
| Injection: | 1 μL |
| Sample: | phosphorothioated RNA 20mer (19 phosphate residues) and phosphorothioated RNA 21mer (20 phosphate residues) |
Analysis of the peak height and area shows that the bioinert YMC-Triart column produces peaks which are double the height and area than obtained on the standard stainless steel column.
A further advantage of coated (‘bioinert’) columns is the reduced requirement for conditioning of the stationary phase in order to obtain a steady state response to difficult compounds. The conditioning mechanism typically involves analyte adsorption onto any active sites within the column to effectively deactivate the column using the analyte itself. Typically several injections are required to reach this steady state response which wastes time and potentially valuable sample. There is a much reduced requirement for this conditioning when columns with deactivated coatings are used as I demonstrated in Figure 4.




Figure 4: Improved column performance from the first injection when using HPLC hardware with bioinert coating (YMC-Accura Triart Bio C4) for the analysis of 100-1000 base unit RNA’s
Figure 4 Conditions;
| Column: | YMC-Accura Triart Bio C4 (3 μm, 300 Å) 100 x 2.1 mm ID |
| Eluent: | A) 50 mM triethylamineacetate (pH 7.0)/acetonitrile (95/5)
B) 50 mM triethylamineacetate (pH 7.0)/acetonitrile (50/50) |
| Gradient: | 9–14%B (0–10 min), 80%B (10–15 min) |
| Flow Rate: | 0.2 mL/min |
| Temperature: | 80 °C |
| Detection: | UV at 254 nm |
| Injection: | 1 μL (0.25mg/mL) |
| Sample: | 100–1,000 bases (CenturyTM-Plus RNA Markers) |
Metal passivation is especially crucial in trace-level analyses where even minor interactions can distort chromatographic performance. The cleaner separation translates into improved resolution and sensitivity, which is vital in pharmaceutical, environmental, and biological applications.
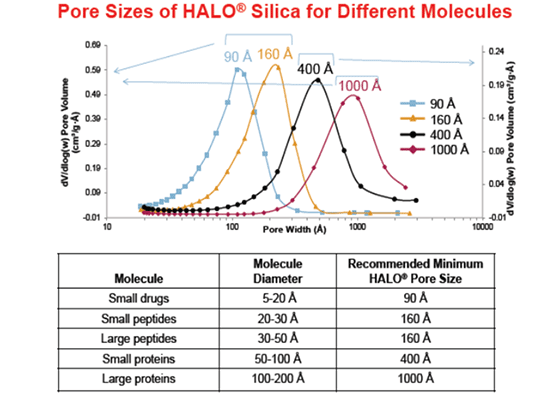
The benefits of the HALO® INERT column hardware from Advanced Material Technologies were observed when comparisons using a 15mer of Oligo dT were undertaken using HALO® OLIGO C18 in both inert and stainless steel hardware. Sample loads of 0.25 to 2 ng were injected on each column. At all concentrations, the area was greater and the tailing was lower with the HALO® INERT column hardware. See Table 1 for the comparative results.
The loading table in Figure 5 shows that as the sample load increases, the difference in peak area generated by the different column hardware decreases. A possible explanation for this may be that as more sample is injected on the stainless steel column, a conditioning effect is observed in which the active metal sites are occupied and cannot bind additional sample. Another observation from this comparison is that the retention time of the Oligo dT 15 mer is reduced with the HALO® INERT hardware compared to the stainless steel hardware. See example chromatograms in Figure 5.


Figure 5: The difference in peak area between coated and uncoated hardware becomes less with increased loading, however peak shape is improved for the coated hardware across all analyte loadings
Figure 5 Conditions;
| Column: | HALO 120A OLIGO C18, 2.7um, 2.1 x 50mm |
| Part Number: | P2A62-402 |
| Mobile Phase A: | 100mM TEAA, Adjusted to pH 8.4 |
| Mobile Phase B: | Acetonitrile |
| Gradient: | 8 -10% 3 mins, 20%B 3.5 mins |
| Flow Rate: | 0.5mL/min |
| Back Pressure: | 146 bar |
| Temperature: | 60C |
| Injection: | 1.0uL |
| Sample Solvent: | 10mM Tris HCL/ 1mM EDTA pH 8.0 |
| Detection: | UV, 254 nm |
The clear retention shift which occurs with the metal-free column and shown in Figure 5, is due to the different nature of column hardware and the different packing procedure. The comparison of the peak areas emphasises that even after pre-conditioning of the stainless steel column a bioinert column is the far better choice.
The use of a bioinert coated YMC-Accura Triart C18 column led to higher intensities and peak areas of four phosphopeptides, compared to the uncoated stainless steel column shown in Figure 6. The high recovery rate of the YMC Accura Triart C18 column also enabled the detection of the challenging phosphopeptide T43pp (compound 3), which contains two phosphate residues and was not detected with the standard column, even after 10 injections.


Figure 6: Improved peak shape and recovery of a highly binding phosphopeptide using YMC-Accura Triart C18 column versus the same stationary phase packed in an uncoated stainless steel column of the same dimensions.
Figure 6 Conditions;
| Column: |
YMC-Accura Triart C18 (1.9 μm, 30 nm) 100 x 2.1 mm ID (bioinert hardware) YMC-Triart C18 (1.9 μm, 30 nm) 100 x 2.1 mm ID |
| Eluent: | A) water + 0.1% formic acid
B) acetonitrile + 0.1% formic acid |
| Gradient: | 0.7%–25%B (0–5 min), 25%B (5–6.6 min), 0.7%B (6.6–8 min) |
| Flow Rate: | 0.6 mL/min |
| Temperature: | 60 °C |
| Detection: | ESI-MS |
| Injection: | 2 μL (10 pmol/μL) |
| Sample: | phosphopeptide enolase standard |
For compounds which are particularly susceptible to adsorption it is sometimes not enough to use a passivated HPLC column. It is sometimes necessary to passivate the HPLC system, especially the components which will contact your analyte such as the autosampler needle, injection valve components and the tubing and fittings between the autosampler and detector (including the sprayer needle if using LC-MS detection). This can be achieved chemically through the instroduction of co-ordination compounds or acidic species, however these methods tend to be unpredictable and reversible. Some manufacturers offer kits to adapt your HPLC system components to less highly adsorbing versions, including the use of MP35N components, and most manufacturers offer off the shelf ‘BioInert’ systems which have been designed and built to offer the highest inertness to problem compounds.


Figure 7: Improved sensitivity and peak shape for phosphate nucleotide coordination compounds obtained when using passivated columns and HPLC systems in various combinations
Figure 7 Conditions - As per Figure 2, PEEK sample loop, PEEK injector port, and PEEK tubing were used to achieve the inertness within the HPLC system.
As seen in Figure 7, for highly problematic compounds, it is sometimes necessary to use both inert HPLC columns and an inert HPLC system in combination, in order to achieve the required chromatographic peak shape and sensitivity.
The requirement for inert hardware applies across all modes of chromatography, including ion exchange and HILIC separations as is demonstrated in figures 8 and 9, which clearly show the improved chromatographic performance of PEEK lined and coated hardware versus the original uncoated stainless steel columns of the same dimensions and stationary phase, acquired on the same HPLC systems.


Figure 8: Comparison of the separation of a 21mer RNA using coated and PEEK lined column hardware using a non-porous polymeric ion exchange stationary phase on a deactivated HPLC system
Figure 8 Conditions;
| Column: | YMC Accura BioPro IEX QF (5 μm) 100 x 4.6 mm ID (bioinert coated hardware)
BioPro IEX QF (5 μm) 100 x 4.6 mm ID (standard hardware) |
| Eluent: |
A) 20 mM Tris-HCl (pH 8.1) |
| Gradient: | 25–40%B (0–15 min), 40%B (15–20 min) |
| Flow Rate: | 1.0 mL/min |
| Temperature: | 60 °C |
| Detection: | UV at 260 nm |
| Injection: | 4 μL (5 nmol/mL) |
| Sample: | 21mer RNA |


Figure 9: Analysis of dT15-35 (left) and rU15-30 (right) using a conventional YMC-Triart Diol-HILIC column (top), a YMC-Triart Diol-HILIC metal-free column (middle) and a YMC-Accura Triart Diol-HILIC column (bottom)
Figure 9 Conditions;
| Column: |
YMC-Triart Diol-HILIC metal-free (1.9 μm, 12 nm) 150 x 2.1 mm ID (PEEK-lined hardware) YMC-Accura Triart Diol-HILIC (1.9 μm, 12 nm) 150 x 2.1 mm ID (bioinert coated hardware) |
| Eluent: | 50 mM ammonium acetate (pH 6.9) Acetonitrile |
| Gradient: | 75–45%B (0–30 min) |
| Flow Rate: | 0.3 mL/min |
| Temperature: | 40 °C |
| Detection: | UV at 260 nm |
| Injection: | 4 μL |
| Sample: | Deoxythymidine oligonucleotide mixtures: dT15-35 (2 μM) and dT40-100 (2 μM) |
After conditioning and analysing the short DNA oligonucleotide mixture dT15-35 and short RNA oligonucleotides rU15-30 were also analysed with a stainless steel column and compared to the metal-free column and the bioinert coated column. Higher sensitivities, peak areas and less peak tailing are achieved using the bioinert column options, even when compared to the PEEK-lined (metal-free) columns.
Whilst much of the discussion on inert hardware has focussed on applications in biopharmaceutical analysis, the unwanted secondary retention effects encountered with metal surfaces within the HPLC flow path and column hardware can be just as pronounced in small molecule applications.
Figure 10 shows the same manufactured lot of HALO 90A C18 2.7um silica was packed into a standard stainless-steel column and a HALO INERT column hardware. Injections of hydrocortisone 21-phosphate sodium salt were made on each column. The difference in chromatography is extremely pronounced. HALO INERT hardware produces approx. 50% smaller tailing factor and 40% increase in peak area, consistent across subsequent repeat injections.


Figure 10: Analysis of small molecules
Figure 10 Conditions;
| Column: |
HALO 90 Å C18, 2.7 μm, 2.1 x 50 mm, INERT HALO 90 Å C18, 2.7 μm, 2.1 x 50 mm |
| Eluent: |
A) 10 mM ammonium formate, pH 3.2 |
| Gradient: | 5-55% B in 3 min |
| Flow Rate: | 0.5 mL/min |
| Temperature: | 30 °C |
| Detection: | UV at 255 nm |
| Injection: | 1.0 μL |
| Sample: | 25 ng/μL hydrocortisone sodium phosphate (90/10 water/methanol) |
Summary
In this technical article we have considered the issue of analyte adsorption and catalysed analyte degradation reactions that can occur on the metal surfaces within HPLC columns.
We have considered the various traditional methods for deactivating metal HPLC hardware including the various pros’ and cons’ of each approach.
The now well-established method of chemical vapour deposition of an inert coating onto metal surfaces is considered alongside a range of applications from the manufacturers YMC and Advanced Material Technologies with their Accura and Halo Inert brands.
It has been demonstrated that, over a wide range of applications but more specifically with applications involving biomolecules containing phosphate moieties, metal columns which have been coated show considerable chromatographic performance advantages over uncoated metal column hardware.
Beyond the column hardware, HPLC systems which have had a similar coating applied to various components also produce superior chromatographic performance.
Anyone working in biopharmaceutical analysis and requiring improved analytical performance should consider the use of coated metal columns to avoid peak shape degradation, lower sensitivity and unwanted degradation reactions.
Whilst the most pronounced improvements are seen with the analysis of biomolecules, small molecule separations in which the analytes have the propensity to bind to metal surfaces can also be significantly improved through the use of coated HPLC column hardware.
References;
[1] Sadek, Paul C.; Carr, Peter W.; Bowers, Larry D.; Haddad, Louis C. A radiochemical study of irreversible protein loss on high-performance liquid chromatography column frits.
Analytical Biochemistry (1985), 144 (1), 29-45
[2] Wakamatsu, Akira; Morimoto, Kentaro; Shimizu, Masao; Kudoh, Shinobu,
A severe peak tailing of phosphate compounds caused by interaction with stainless steel used for liquid chromatography and electrospray mass spectrometry.
Journal of Separation Science (2005), 28 (14), 1823-1830
[3] Liu, Suya; Zhang, Cunjie; Campbell, J. Larry; Zhang, Haixia; Yeung, Ken K.-C.; Han, Victor K. M.; Lajoie, Gilles A., Formation of phosphopeptide-metal ion complexes in liquid chromatography/electrospray mass spectrometry and their influence on phosphopeptide detection. Rapid Communications in Mass Spectrometry (2005), 19 (19), 2747-2756
[4] Sakamaki, Hiroshi; Uchida, Takeharu; Lim, Lee Wah; Takeuchi, Toyohide, Evaluation of column hardware on liquid chromatography-mass spectrometry of phosphorylated compounds. Journal of Chromatography A (2015), 1381 (), 125-131
[5] Hsiao, Jordy J.; Staples, Gregory O.; Stoll, Dwight R., Troubleshooting LC separations of biomolecules, part I: background, and the meaning of inertness. LCGC North America (2020), 38 (3), 146-150
[6] Euerby, M. R.; Johnson, C. M.; Rushin, I. D.; Tennekoon, D. A. S. S. Investigations into the epimerization of tipredane ethylsulphoxide diastereomers during chromatographic analysis on reversed-phase silica II. The involvement of metals in commercially available C18 silicas. J. Chromatogr. A 1995, 705, 229– 245
[7] Winter, D.; Seidler, J.; Ziv, Y.; Shiloh, Y.; Lehmann, W. D. Citrate Boosts the Performance of Phosphopeptide Analysis by UPLC-ESI-MS/MS. J. Proteome Res. 2009, 8, 418– 424.
[8] Siegel, D.; Permentier, H.; Bischoff, R. Controlling detrimental effects of metal cations in the quantification of energy metabolites via ultrahigh pressure-liquid chromatography-electrospray-tandem mass spectrometry by employing acetylacetone as a volatile eluent modifier. J. Chromatogr. A 2013, 1294, 87– 97.
[9] Hsiao, J. J.; Potter, O. G.; Chu, T.-W.; Yin, H. Improved LC/MS Methods for the Analysis of Metal-Sensitive Analytes Using Medronic Acid as a Mobile Phase Additive. Anal. Chem. 2018, 90, 9457– 9464.
[10] https://sisw.cz/cz/downloads/polymer-resistance.pdf (accessed May 21st 2025)
[11] Kromidas, S., Ed, The HPLC Expert II. Wiley-VCH, 2017
[12] https://www.silcotek.com/blog/the-perfect-frit.-how-to-protect-your-hplc-column-and-results (accessed May 21st 2025)
[13] Martin Gilar, M., DeLano, M., Gritti, F., Mitigation of analyte loss on metal surfaces in liquid chromatography, Journal of Chromatography A 1650 (2021) 462247
[14] Smith D.A., Mattzela J.B., Silvis P.H., Barone G.A., Chemical Vapor DepositionCoating, Article, and Method, USPTO, Application US 13/504,533 (2012).
[15] Smith D.A., Mattzela J.B., Silvis P.H., Higgins M.E., Wear Resistant Coating, Article, and Method, USPTO, Application US 13/876,328 (2013).