Silica Gel as Chromatographic Support
High performance liquid chromatography (HPLC) is predominantly based on stainless steel columns tightly packed with microscopic particles supporting a stationary phase. A great number of support materials have been tested over the decades, and many have fallen out of use along the way. Silica gels, however, have stood the test of time and are the support material of choice for many types of liquid chromatography.
Silica gel is a remarkable material, with several properties that make it a desirable support for chromatographic applications:
-
High purity (type “B”) silica can be synthesised from monomeric precursors such as tetraethoxysilane (TEOS)
-
Spherical particles can be manufactured with tightly controlled dimensions
-
High porosity, and the ability to control average pore size
-
Mechanical strength, which enables efficient packing
-
Compatibility with a wide range of aqueous and organic solvents
-
Above all, the ability to modify surface chemistry by bonding organic moieties to surface silanol groups


Figure 1: Chemical modification of silica surface by SN2 reaction of a silanol group onto an alkyl siloxane (-Si(Me)2-R) reagent. Through this reaction, any stationary phase can be bonded to the particle surface. When R = (CH2)17CH3, the resulting stationary phase is called C18 (octadecylsilane).
Limitations of Silica Gel
While silanol groups confer the flexibility to bond stationary phases, endcapping, and other chemical modifications, they are also the cause of a major weakness of silica particles: limited resistance to extreme pH
- At low pH (typically, below pH 2) the siloxane bond between silica and the organic modification can be hydrolysed, resulting in the loss of stationary phase
- At high pH (approximately above pH 8) silica becomes soluble, compromising the mechanical integrity of the particle
Another important limitation in acidic mobile phases is that silanol groups are partially deprotonated, and create a negative charge on the silica surface. When analysing basic compounds, which in that environment tend to be protonated, strong ionic interactions tend to produce distorted (tailing) chromatographic peaks:


Figure 2: Ionic interactions between a protonated basic analyte (positively charged) and deprotonated silanol group (negatively charged) in mildly acidic to neutral conditions.
Since many important drugs are basic, the pharmaceutical industry has historically driven column manufacturers to develop solutions to these analytical problems. Endcapping (derivatising unreacted silanols with trimethylsilane (TMS) or a similar reagent) has been a widespread strategy. Another common approach has been to use ion pair reagents such as trifluoroacetic acid (TFA) to neutralise positively charged analytes.
Both endcapping and ion pairing, however, have technological and practical limitations. The most efficient way to analyse most basic compounds is by suppressing ionic interactions in alkaline mobile phases, above the pKa of the compound. Unfortunately, most silica columns cannot sustain continued operation at this high pH, and particle erosion can destroy the column in a matter of hours.


Figure 3: Analysis of drugs of abuse by reversed phase in acidic (pH 2.6, top), neutral, and increasingly basic (pH 10.9, bottom) mobile phases on HALO® Elevate C18. Note the increased retention and peak shape improvement for basic compounds (peaks 3, 4, 5, 6). Adapted from [1].
Rise of the Hybrids
In an effort to solve the problem of high pH stability, Waters Corporation introduced in 1999 a novel approach to the synthesis of chromatography silica particles: by combining TEOS (precursor of type “B” silica) with methyltriethoxysilane (MTEOS) Waters produced the first generation of “organic-inorganic hybrid particle technology” columns.
This “hybrid silica” support reduced tailing for basic compounds and improved resistance to high pH, but it also resulted in fragile particles that crumbled at high pressures. A second generation of hybrid silica followed in 2004, this time incorporating bis(triethoxysilil)ethane (BTEE) as the organosilane monomer:


Figure 4: Second generation organic-inorganic hybrid silica.
This packing material, aptly called “bridged ethyl hybrid”, became a commercial success. It could be used up to pH 12 for extended periods of time and was available with C18, C8, and Phenyl stationary phases. Finally, chromatographers seemed to have it all: particles with structural strength, chemical inertness, and durability at high pH.
The Other Revolution
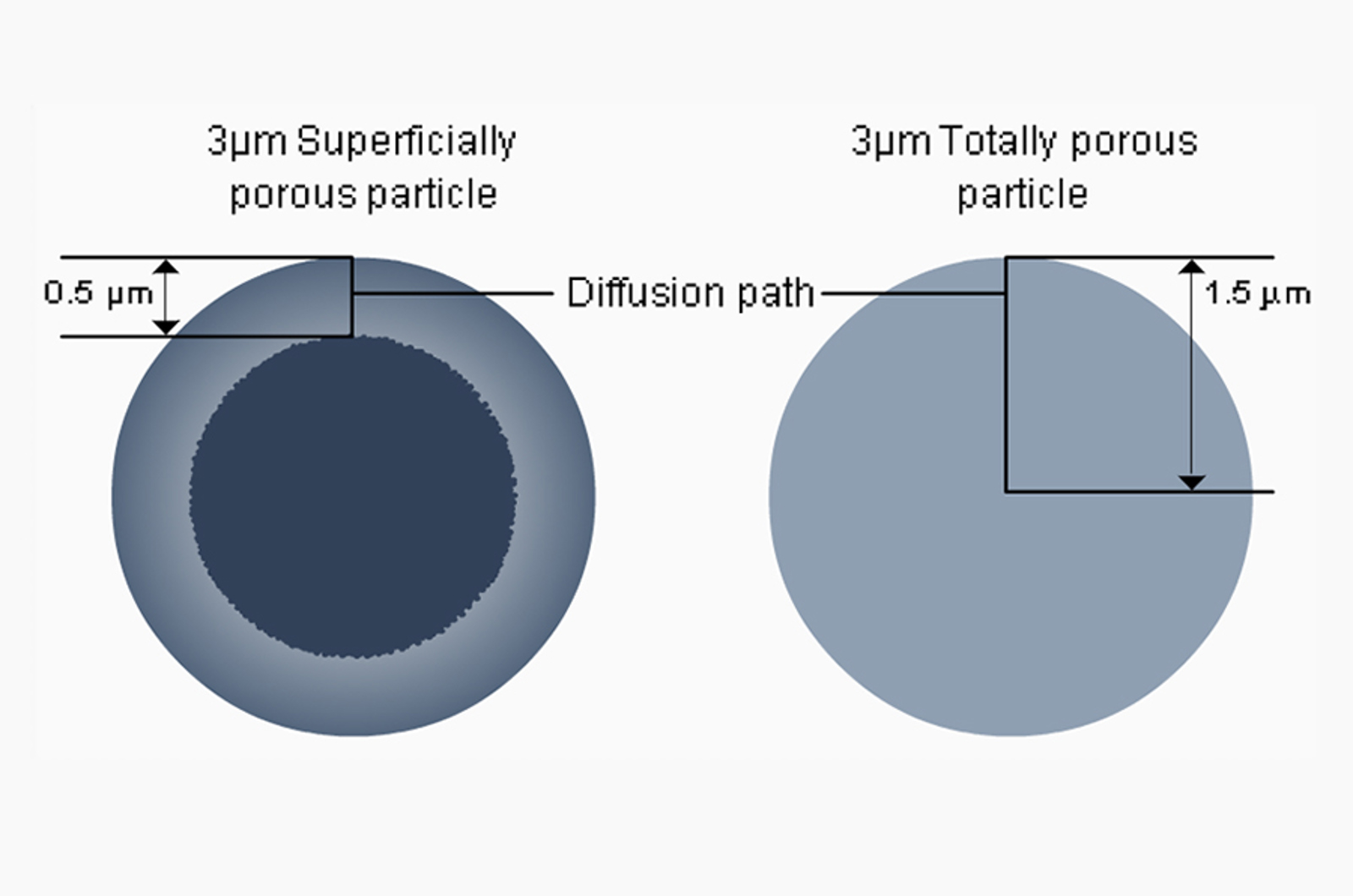
Simultaneously, in 2006, Advanced Materials Technology (AMT) pioneered superficially porous particles (SPP), or fused core silica: a new type of silica particle technology. SPP are synthesised from high-purity type “B” silica in two stages. In stage one, reaction conditions promote the growth of non-porous, spherical particles. In the second stage, conditions are adjusted to grow a layer of porous silica over the solid core.
The resulting particle was called HALO®, and had a solid core of precisely controlled diameter (1.7μm) surrounded by a shallow (0.5μm) porous shell. The monodisperse particle distribution allowed for very efficent packing and minimal linear dispersion, whilst the shallow pore shell enabled fast mass transfer kinetics between eluent and stationary phase.


Figure 5: Anatomy of a superficially porous HALO® particle. The inner core is solid, with a tightly controlled diameter of 1.7μm. The outer shell is fully porous with a thickness of 0.5μm and 90Å pore size. More recently, Halo particles of different diameters, shell thicknesses, and pore sizes have been released for specialised applications.
HALO® columns with 2.7μm SPP particles competed favourably with early sub-2 micron fully porous particles (FPP). Both technologies exhibited similar van Deemter curves and produced fast, highly efficient separations. However, where sub-2 micron particles generated unprecedented pressures that necessitated specialised UHPLC systems, SPP particles operated at much lower pressures, compatible with the prevailing HPLC systems of the time.


Figure 6: Van Deemter curve for a superficially porous particle (2.7μm HALO®), compared to fully porous particles (FPP) of various sizes. Halo 2.7μm (light blue) outperforms classical 5μm (dark blue) and high efficiency 3.5μm (orange) particles and is comparable to much smaller 1.8μm FPP (grey), thanks to very low longitudinal dispersion (B term) and mass transfer characteristics (C term). Adapted from [2]
HALO® Elevate C18
Despite their impressive performance, SPP particles were still limited to eluents below pH 8, and hybrid silica seemed to have the upper hand for analysing active basic compounds. While the first stage of manufacturing SPP uses inorganic type “B” silica, it is possible to introduce organic-inorganic silanes into the second stage, protecting the porous shell from alkaline attack. This principle was applied to the development of the latest HALO® particle: Halo Elevate.
Table 1: HALO® Elevate C18 technical specifications
| Particle sizes | 2.7μm and 2.0μm |
| Shell thickness | 0.5μm |
| Pore size | 120Å |
| pH stability | 2 – 12 up to 60°C |
| Stationary phase | C18 (USP L1) |
| Lengths | 30, 50, 100, 150, 250 mm |
| Internal diameters | 1.5, 2.1, 3.0, 4.6 mm |
HALO® Elevate has the structural characteristics of HALO® particles, but the porous shell incorporates an organic-inorganic layer. The particles can resist hundreds of hours of continued operation at pH 10 and temperatures as high as 60°C:


Figure 7: HALO® Elevate C18 tested over 500 injections (20,000 column volumes) at pH 10 and 60°C. 1: uracil, 2: acenaphthene, 3: amitriptyline. Column: HALO® 120Å Elevate C18 2.7μm, 2.1 × 50mm PN: 92272-402. Adapted from [1].
On-column concentration of basic compounds is severely restricted in low pH conditions, due to peak distortion and rapid loss of resolution. This distortion is in part explained by electrostatic repulsion between cationic analytes, causing the analyte band to expand. Under high pH conditions, however, basic compounds become neutral and column loadability improves significantly:


Figure 8: Loadability studies of trimipramine on HALO® Elevate C18 in basic pH conditions (green) and HALO® C18 in acidic pH conditions (red). Note the rapid decrease in theoretical plates as increasing amounts of trimipramine are injected under acidic conditions. Adapted from [3].
Combining the excellent van Deemter characteristics of HALO® particles, robustness at high pH, and enhanced sample loadability, HALO® Elevate 120Å C18 2.7μm particles can outcompete sub-two micron hybrid silica particles, and even exceed them in long-term stability at up to pH 12 and temperatures up to 60°C.
With the recent introduction of 2μm HALO® particles and ultra-low dispersion 1.5mm ID columns optimised for LC-MS applications, the efficiency of HALO® Elevate 120Å C18 is only really limited by extra-column dispersion occurring in the LC system:


Figure 9: Separation of omeprazole, related substances, and impurities on HALO® Elevate 120Å C18 2μm, 2.1 × 100 mm (PN: 91272-602). Eluent pH: 10.62. Column temperature: 60°C. Peaks 1: Related substances F and G, 2: rel. subst. B, 3: rel. subst. E, 4: rel. subst. A, 5: impurity B, 6: omeprazole, 7: imp. H, 8: N’-methyl omeprazole isomer 1, 9: N’-methyl omeprazole isomer 2, 1: imp. C. Adapted from [1].
Epilogue: Is One Column Better than Many?
Hybrid silica C18 columns seem a no brainer: use one single column to analyse acidic, neutral, and basic compounds across the widest range of pH conditions. Most column manufacturers, however, have opted for traditional type “B” silica (either FPP or SPP) and developed subtle variations of C18 for acidic, neutral, or basic conditions. This is the strategy pursued by AMT, who have no fewer than five C18 modalities:
Table 2: Current modalities of C18 stationary phases in the AMT HALO® catalogue.


Why would AMT choose this strategy? Is there any advantage in developing five C18 columns, instead of a single one for all conditions?
At Element Lab Solutions, we often use the Hydrophobic Subtraction Model (HSM) and the enhanced HSM 2 [4], to help us analyse similarities and differences between columns and make recommendations to our customers. The following table shows the HSM2 descriptors of the five HALO® C18 columns, plus the bridged ethylene hybrid C18 from Waters Corporation:
| Column | H | A | B | C | V | D |
| Waters "Hybrid" C18 | 0.9980 | -0.1200 | -0.0100 | 0.1850 | 0.0650 | 0.0230 |
| HALO® LPH C18 | 1.0017 | 0.3145 | 0.1195 | 0.1963 | -0.1816 | -0.0914 |
| HALO® C18 | 1.0190 | -0.0570 | -0.0190 | -0.0360 | -0.0090 | -0.1190 |
| HALO® AQ-C18 | 1.0610 | 0.1620 | -0.0810 | 0.2130 | -0.0020 | 0.0770 |
| HALO® Elevate C18 | 1.0306 | -0.0956 | -0.0311 | 0.2613 | 0.0420 | -0.0006 |
| HALO® PCS C18 | 1.1308 | -0.0678 | 0.0354 | -0.1593 | 0.0117 | 0.2479 |
Hydrophobic interaction (H) is dominant and very similar across all six columns, which is to be expected as they incorporate a C18 modifier. Comparisons can be simplified by normalising each of the remaining descriptors with respect to H (A’ = A / H, B’ = B / H, etc.) The resulting normalised descriptors can be overlaid in a “spider plot”:


Figure 10: Comparison of six columns using HSM2 stationary phase descriptors. H: hydrophobicity, A: hydrogen bond acidity, B: hydrogen bond basicity, C: ion exchange capacity, V: steric selectivity, D: dipole interaction. “Hybrid” C18 refers to the second generation organic-inorganic hybrid silica C18 from Waters Corporation.
This diagram highlights the differences in selectivity most strongly linked to eluent pH, analyte polarity, and secondary interactions. It shows that HALO® Elevate C18 (purple) has a very similar profile to Waters “Hybrid” C18 (dark area). This should not be surprising, given that both columns have similar surface chemistries. It also shows how radically different HALO® LPH C18 (orange) is: this column is optimised for hydrogen bonding and ionic interactions, and excels at separating organic acids, acidic pesticides, and polar compounds at the opposite end of the pH scale.
It is also important to notice how different HALO® C18 (dark green) is from HALO® AQ-C18 (light blue). The former is a classic endcapped C18, designed to suppress secondary interactions. The latter is designed to enhance ion exchange, dipole-dipole, and hydrogen bonding, critical for retaining polar compounds in highly aqueous conditions.
Finally, it is apparent that HALO® PCS C18 (light green) is very different from all the others. This column incorporates a positively charged endcapping modifier, which confers the stationary phase a completely different set of interaction mechanisms. But perhaps that is a tale for another day.
The HSM2 analysis clearly demonstrates the advantage of having many C18 chemistries: a “universal” column cannot change its stripes, it will always interact in the same way irrespective of the type of analyte, eluent, or pH. A toolkit of slightly different columns, each designed for a compound class or eluent pH range, not only provides flexibility and optimised performance under each set of conditions, but also the degree of orthogonality that method developers need when tackling the most complex separations.
References
[1] HALO® Elevate C18. Taking Separations to a Higher Level, Advanced Materials Technology, 2025.
[2] HALO® Catalogue, Advanced Materials Technology, 2018.
[3] C. McHale, HALO® Elevate: Universal Column for Small Molecule Method Development, Advanced Materials Technology, 2024.
[4] Stoll, Dwight, et al., “Improvements in the predictive accuracy of the hydrophobic subtraction model of reversed-phase selectivity,” J. Chrom. A, vol. 1636, p. 461682, 2021.