Introduction
Advanced Materials Technology (AMT), manufacturers of Fused-Core® silica particles for HPLC, launched two landmark column ranges in 2024: HALO® ELEVATE, designed to operate at high pH and temperature, and HALO® PCS (Positive Charged Surface) for improved peak shapes with basic compounds. They also launched new a surface-passivated range (HALO® Inert) which prevents unwanted interactions between chelating compounds and stainless-steel hardware. With the new introductions, AMT has marketed roughly 30 stationary phases. And innovation doesn’t stop here: new optimised columns for large nucleotides seem to be coming soon.
With HALO® approaching its 20th anniversary, we wanted to pause and reflect on the current range of chemistries. Do we need so many? Are they really all that different? Do they all serve a purpose? In this article, we take a deep dive into the world of HALO® reversed phase columns.
HALO® HPLC Columns
In 1970, a young chemist at Du Pont de Nemours (Wilmington, DE) called Jack Kirkland filed a patent application, “Superficially Porous Supports for Chromatography” (1) describing a method for producing pellicular particles of uniform size by coating glass beads with “colloidal particles of a given size and ionic charge… a single monolayer at a time”.


Figure 1: “Fig. 1” in Kirkland’s US Patent 3505785, describing the structure of a superficially porous particle.
That same year, Kirkland and du Pont colleague J. de Stefano published a paper in the Journal of Chromatographic Science demonstrating the application of these particles to GC and LC separations (2). Kirkland recalled this early work during a 2016 interview with Gert Desmet: “These particles were commercialized by DuPont as Zipax in 1968 and Permaphase in 1970. With the development of the much smaller and more efficient porous silica microspheres such as Zorbax in the early 1970s, the larger Zipax particles quickly lost favor. More than 20 years passed before there was a rebirth of SPP” (3).
The “rebirth of SPP” culminated in the incorporation of AMT in 2005 and the launch, a year later, of the first sub-3μm superficially porous particle: the HALO® C18 2.7μm.


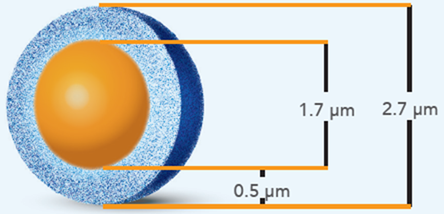
Figure 2: Illustration of a HALO® Fused-Core particle consisting of a solid, non-porous, 1.7μm silica core with a 0.5μm layer of fully porous silica nanoparticles. Adapted from https://HALO®columns.com.
The chromatographic benefits of HALO® particles have been extensively discussed elsewhere (4 - 6). In short, their efficiency is comparable to that of sub-2μm, fully porous particles (FPP), but they are far less sensitive to practical problems such as blocking and over-pressure.


Figure 3: van Deemter curves of Fused-Core particles against fully porous particles of different diameters. Notice the close performance of HALO® 2.7μm to that of smaller 1.8μm FPP particles. Adapted from https://HALO®columns.com.
In the intervening years, the HALO® family has been constantly expanding with new chemistries, particle morphologies, application-specific solutions, and hardware innovations:


Hydrophobic Subtraction Model
The Hydrophobic Subtraction Model (HSM), developed by Lloyd Snyder, Peter Carr, John Dolan, and coworkers, was published over a series of articles in Journal of Chromatography A, starting in 2002 (7). The aim of the model was very practical: to develop a quantitative system to determine whether two given columns, under the same analytical conditions, would produce equivalent, similar, or orthogonal separations.
According to HSM, the retention factor of a solute on a given stationary phase can be modelled as:
log k = log kref + η′H + σ′S + β′A + α′B + κ′C + e
Where kref is the retention factor of a reference compound. Greek symbols η′, σ′, β′, α′, κ′ are solute hydrophobicity, molecular shape, hydrogen bonding basicity and acidity, and ion exchange capacity.
The letters quantify the hydrophobicity, shape selectivity or steric hindrance, hydrogen bonding (donor and acceptor), and ion exchange capacity of the stationary phase. The term is the model error.
A column is characterised by determining its . This is done by analysing a mixture of sixteen compounds of well-established properties, under standardised conditions (60mM phosphate buffer, pH 2.8/MeCN 50:50), and deconvolving the column descriptors from the empirical retention factors.


Figure 5: Sixteen chemical probes used by HSM to characterise reversed phase chromatographic columns. The compounds were carefully selected to include a broad range of functional groups and chemical properties. Ethylbenzene is the reference compound. Adapted from (8).
HSM has been used to catalogue more than 750 columns, including some chemistries (phenyl, PFP, CN, polar-embedded...) that extend far beyond the original boundaries of the model, C18. A discussion with the curators of the HSM database about whether the model was still valid for these new stationary phases, resulted in the publication of an “enhanced” HSM, which became known as HSM2. The new model recalculated the key stationary phase-analyte interaction descriptors, removed steric hindrance in favour of solute size, and introduced solute dipolarity as a new parameter (8):
log k = log kref + hH + bA + aB + kC + vV + dD + e
HSM2 predicts the correct selectivity (solute retention order) better than the original model, with Kendall τ correlations above 0.86, and reduced by 65% the standard error. This translates into more reliable measures of equivalence/orthogonality when researching columns for our customers. Highly accurate solute retention factors enable us to generate “predicted chromatograms”, which makes column comparisons easier to interpret (9).
HALO® Reversed Phase Columns in the HSM Database
The current offering of HALO® columns in reversed phase includes some thirty types, including columns for pharmaceutical, industrial chemistry, and food analysis, solutions for environmental analysis (PAH, PFAS), and wide-pore columns with optimised shell thickness for characterising peptides, proteins, and oligonucleotides.
A good number of these have been characterised by the HSM method and can be found in the database:


Figure 6: HALO® stationary phases currently catalogued in the HSM and HSM2 databases.
The HSM2 database gives us a unique opportunity to evaluate and “quantify” the chemical interactions offered by each stationary phase. Further, we can use predicted chromatograms to visually compare the chromatography produced by each column under identical analytical conditions.
C18 Columns
Most manufacturers design a variety of C18 columns with different surface coverages, carbon loadings, etc., to offer a wide range of hydrophobicities. The HALO® columns, by contrast, have moderate, very similar hydrophobicities:


Figure 7: Hydrophobicity of six L1 HALO® columns, in absolute values (labels) and relative to the “baseline” HALO® C18 (vertical axis). These values lie approximately in the second quartile of the HSM2 database, making these columns only moderately hydrophobic.
Remarkably, these H values do not correlate well with carbon loads (C%, see Figure 6). HALO® PCS C18 and HALO® AQ-C18 have the highest H values, despite having lower C% than HALO® C18. Both columns incorporate unique endcapping groups, designed to enhance peak shape and loading capacity for basic compounds (PCS C18) and retention in 100% aqueous eluents (AQ-C18). The data suggests that C% may not be a perfect indicator of hydrophobicity, and that endcapping chemistry plays an important role in analyte retention.
In C18 stationary phases, hydrophobic interaction (H) dominates over all other mechanisms. Despite being quantitatively lower, however, the rest of descriptors (A, B, C, etc.) are the most informative, as they explain the differences in selectivity (elution order) between columns. To better visualise these parameters, we often “normalise” (divide each value by H) and graph them on a radar (also known as Tanaka) plot:


Figure 8: Normalised Tanaka plots for the six L1 HALO® columns.
The Tanaka plots reveal enormous variability for all parameters, except V/H. Similar V/H values are expected, since V is mostly influenced by C% and stationary phase type. The rest of parameters, however, are extremely interesting from a method development point of view: each column seems to have optimised hydrogen-bonding, cation exchange, and dipole-dipole interactions according to its intended application.
HALO® AQ-C18 and HALO® C18
The polar stationary phase of HALO® AQ-C18, for example, is designed to improve retention in aqueous eluents. The chemistry is proprietary, but it clearly has higher hydrogen-bond acidity, dipolar character, and cation exchange capacity than HALO® C18:


Figure 9: Normalised Tanaka plot of HALO® 90Å AQ-C18 versus HALO® 90Å C18.
Comparing HALO® AQ-C18 to HALO® C18 shows the chromatographic effects of these chemical modifications: retention is increased across all polar compounds, and resolution is improved between closely related pairs.


Figure 10: HSM2 calculated chromatograms for HALO® C18 (red, top trace) and HALO® AQ-C18 (blue, bottom trace).
Note how AQ-C18 improves the separation between nortriptyline and amitriptyline, possibly due to differences in hydrogen-bonding between secondary and tertiary amines. It also exploits the different polarisability in pi systems to improve the separation between cis- and trans-chalcone.
HALO® LPH-C18 and Elevate C18
Another interesting comparison is that of HALO® LPH-C18 and Elevate C18, which were designed for complementary applications: LPH-C18 incorporates a di-isobutyl-octadecylsilane group, which shields the stationary phase from acidic hydrolysis. This column is not endcapped. In Elevate C18, on the other hand, the silica surface is heavily endcapped using organo-silane technology to increase resistance to highly alkaline eluents. Tanaka plots of both phases beautifully mirror their opposite characters:


Figure 11: HALO® C18, HALO® LPH-C18, and HALO® Elevate C18. Note the strong hydrogen-bonding ability of LPH-C18 due to free silanol groups, practically inexistent in the heavily endcapped Elevate C18. Elevate C18 exhibits stronger dipole interactions and steric selectivity, consistent with an extensive three-dimensional network of Si–O–Si bonds on the surface.
The differences between these stationary phases are noticeable when we analyse the calculated chromatograms:


Figure 12: Predicted chromatograms for HALO® LPH-C18 (red, top trace) and HALO® Elevate C18 (blue, bottom trace).
Mefenamic acid, cis- and trans-chalcone, ethylbenzene, toluene, and butylbenzoic acid are well retained on LPH-C18. These are some of the bulkiest HSM probes, and would tend to be excluded (less retained) on Elevate C18. Elevate C18, on the other hand, successfully resolves anisole from phenylpentanol. These compounds have similar polarities, but differ in the length of the alkyl chain and, therefore, in hydrodynamic radius.
HALO® PCS, Peptide ES-C18, and C18
HALO® PCS C18 is one of the latest chemistries developed by AMT. PCS (Positive Charge Surface) refers to a second surface ligand (in addition to ODS) that contains an amine group. In acidic eluents, amine groups become protonated, coating the particle surface with positive charges. These charges inhibit secondary interactions with basic compounds and improve peak symmetry. At basic pH, the ligands become neutral and the charges disappear, but the amine groups provide dipole-dipole and hydrogen-bond interactions with the analyte.
HALO® Peptide ES-C18 is part of AMT’s Bioclass range. It is designed for peptide analysis, with 160Å pore particles. The stationary phase is virtually identical to that of HALO® LPH C18, and the surface is not endcapped.
Peptide ES-C18 has the lowest H parameter of all HALO® columns currently in the database. This is typical of particles with wide pores, which result in lower surface areas (only 22 m2/g) and therefore less stationary phase per particle. The large pores should provide easy access to surface silanol groups, and we would expect this column to have great cation exchange, hydrogen bonding, and dipole-dipole capacity. The Tanaka graphs confirm these predictions:


Figure 13: HALO® C18, HALO® PCS C18, and HALO® Peptide ES-C18.
An analysis of the HSM2 chromatograms reveals that HALO® PCS C18 and HALO® C18 are remarkably orthogonal, with four peak inversions. Notably, butylbenzoic acid and mefenamic acid are retained much more strongly on HALO® PCS C18 than they are on HALO® C18. This is easily explained by the presence of positive surface charges retaining the negatively charged acids by anion exchange:


Figure 14: HSM2 comparison of HALO® C18 (green, top trace), HALO® PCS C18 (red, middle trace), and HALO® Peptide ES-C18 (blue, bottom trace).
It is worth pointing out that anion exchange was not modelled into HSM2 (only cation exchange by silanol groups was considered), since the database contained no stationary phases with positively charged groups at the time. But the remarkable flexibility of HSM2 overcomes this limitation: HALO® PCS C18 indeed has a negative C descriptor, which successfully models the “missing” mechanism.
PCS phases were originally designed to improve peak shapes for basic compounds in low ionic strength mobile phases. However, their intrinsic ion exchange capacity makes them an effective tool for analysing anionic compounds too:


Figure 15: LC-MS analysis of short- and long-chain PFAS on HALO® PCS C18. Adapted from (10).
In Figure 14, Peptide C18 shows lower overall retention, which is typical of large pore size particles. Out of curiosity, we wanted to assess the similarity between HALO® LPH-C18 and HALO® Peptide ES-C18. If the stationary phases were identical, as we assumed above, their HSM2 chromatograms should be very similar. Figure 16 confirms our suspicion: accounting for the lower retention, and the lower resolution due to 160Å not being optimal for small molecules, selectivity on both columns is virtually identical.


Figure 16: comparison of HALO® LPH-C18 (red, top trace) and HALO® Peptide ES-C18 (bottom, blue trace).
Phenyl Columns
Four HALO® “phenyl” phases are catalogued in the HSM database: biphenyl, phenyl-hexyl, PCS phenyl-hexyl (all classified as USP L11) and PFP (USP L43). The Tanaka plots show less variability than we have seen between C18 phases:


Figure 17: Normalised Tanaka plot of the four Phenyl-type stationary phases in the HSM database. HALO® C18 (dark grey) added for reference.
HALO® Biphenyl and PFP
Phenyl and PFP phases often demonstrate orthogonal selectivities. This is generally explained by the benzene pi system being electron-rich in the former and electron-poor in the latter. The different electronic densities of HALO® Biphenyl and HALO® PFP are illustrated in a striking way by our Tanaka plots:


Figure 18: HALO® Biphenyl versus HALO® PFP.
Descriptors B, C, V, and D are very similar on both columns, and one would expect nearly identical chromatography based on these. However, A – the ability to accept electrons – is extremely low for Biphenyl and high for PFP, exactly as molecular orbital theory would predict. The HSM2 chromatograms show a textbook example of orthogonal stationary phases:


Figure 19: HSM2 chromatograms for HALO® Biphenyl and HALO® PFP. Note the multiple peak inversions, demonstrating a high degree of orthogonality. Biphenyl also exhibits higher hydrophobic retention (H) thanks to having two phenyl rings.
This figure clearly demonstrates the value of incorporating a HALO® PFP column into our method development kits: with minimal or no change in eluent conditions, these columns can easily resolve coeluting pairs that might otherwise require weeks of method optimisation.
HALO® Phenyl-Hexyl and PCS Phenyl-Hexyl
We demonstrated earlier that the introduction of PCS derivatisation onto the HALO® C18 stationary phase produced remarkable results. Is that also true when PCS is added to an L11 chemistry, such as the popular phenyl-hexyl?
The Tanaka plots suggest that the answer is affirmative: Both phases have complementary hydrogen-bond (A, B) parameters. HALO® Phenyl-Hexyl has a strong anion exchange character, which is completely missing in its PCS counterpart. Finally, PCS Phenyl-Hexyl seems to have higher steric and dipole-dipole selectivity.


Figure 20: HALO® Phenyl-Hexyl and HALO® PCS Phenyl-Hexyl.
The HSM2 reconstructed chromatograms confirm our expectations, with subtle but significant differences in selectivity. Like its PCS C18 counterpart, PCS Phenyl-Hexyl strongly retains acidic probes due to low-pH anion exchange interactions.


Figure 21: HSM2 comparison of HALO® Phenyl-Hexyl and HALO® PCS Phenyl-Hexyl.
Conclusion
AMT have developed a comprehensive catalogue of reversed phase columns, since the launch of HALO® 2.7μm C18 two decades ago. This variety of products is welcome news for LC and LC-MS method developers who, faced with increasingly complex analytical problems, need to identify the right column for each job, quickly and with confidence.
The enhanced Hydrophobic Subtraction Model (HSM2) can help uncover the design principles and practical benefits of each column. Rather than running through a list of statistics, names, and stationary phases, we have used HSM2 to illustrate how each HALO® column has been cleverly optimised for its intended application. More importantly, the visual tools provided by HSM2 help understand each column, explore how they compare to alternative solutions, and accurately predict their real-world chromatographic behaviour.
The HALO® family will continue to grow, and we are excited about the innovations these future columns will bring to our labs. We look forward to helping you find the perfect one to tackle your next separation challenge.
References
- J. J. Kirkland, US Patent 3505785 (1970)
- J. J. Kirkland, J. J. DeStefano, J. Chromat. Sci. 8 (1970) 309-314
- G. Desmet, LCGC Europe 26, 8 (2013) 438-443.
- Choosing Between Fully Porous and Superficially Porous Particles in HPLC: Performance, Pressure, and Practicality.
- Superficially porous particles.
- Core-Shell particles.
- N.S. Wilson, M.D. Nelson, J.W. Dolan, L.R. Snyder, R.G. Wolcott, and P.W. Carr, J. Chromatogr. A 961 (2002) 171–193
- A New View of Reversed Phase HPLC Selectivity.
- D.R. Stoll, T.A. Dahlseid, S.C. Rutan, T. Taylor, J.M. Serret, J. Chromatogr. A 1636 (2020) 702 461682
- Advanced Materials Technology, HALO® PCS Family Brochure, AMT24_PCS_Rev1 (2025)