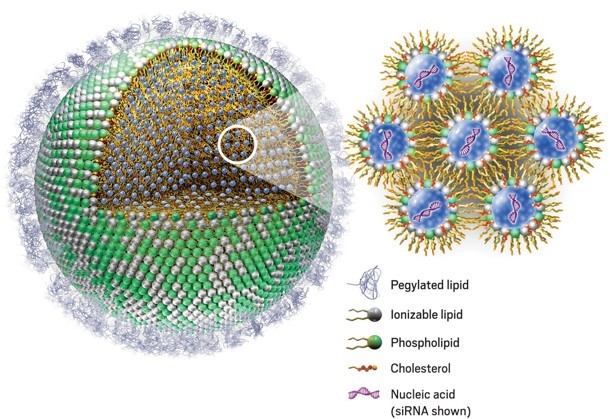
Phospholipids are amphiphilic molecules containing a hydrophilic “head” and two hydrophobic “tails”. In aqueous solution, phospholipids spontaneously arrange into continuous “monolayers” or “bilayers”, in such a way that the polar heads are exposed to the aqueous medium, while the hydrophobic tails are shielded from it. This arrangement is the foundation of all living cell and organelle membranes, as well as that of synthetic lipid-based nanoparticles, or lipid nanoparticles (NLPs).


Figure 1: Lipid nanoparticle (LNP) encapsulating small interfering RNA (siRNA). Phospholipid polar heads (green) are arranged on the outside of the particle, in contact with the aqueous environment, whilst the hydrophobic tails (yellow) remain in the inside of the particle. Inside the LNP, bilayers of phospholipids emulsify nanodrops of aqueous buffer (blue) containing siRNA molecules. Cholesterol (red) adds stability. Pegylated lipids on the outer layer prevent the LNP from being detected by the immune system, allowing the particles to reach their target tissue. Through a mechanism called endocytosis, tissue cells enclose the LNP into an endosome, which is transported into the cytoplasm. The acidic pH inside the endosome protonates the ionisable lipids (white). This disintegrates the LNP and releases the siRNA into the cytoplasm, where it can carry out its function. Adapted from [1]
LNPs have raised enormous interest as novel drug delivery systems, especially for RNA-based therapeutics after the technology was applied to the Moderna and Pfizer-BioNTech Covid-19 vaccines. LNPs are typically spherical, between 10nm and 1μm in diameter, and are composed of phospholipids, cholesterol, PEGylated lipids, and ionisable lipid molecules. The nature and proportions of the constituent lipids are carefully engineered to reduce the degradation of the therapeutic payload, enhance biological efficacy and specificity, and minimise cytotoxicity.
Like all complex formulations, LNP-based therapeutics are subject to a number of critical quality attribute (CQA) checks. The FDA published in 2022 a set of risk-based guidelines to assess the CQAs of these products [2] which includes, among others, chemical composition, average particle size and polydispersity, shape and morphology, and chemical stability. Many of these CQA are measured using different modes of liquid chromatography. Element Lab Solutions offers a comprehensive set of HPLC columns, methods, training and technical support for the characterisation of these important drug products.
Chemical Composition on Agilent Poroshell CS-C18
The chemistry and relative proportion of the constituent lipids determine many of the critical performance characteristics of LNPs. In application note 5994-5031EN [3], Agilent Technologies demonstrate the characterisation and quantitation of lipid components using a Poroshell CS-C18 column:


Figure 2: chromatographic analysis of LNP components on Poroshell CS-C18.
LNP component structures:


Poroshell CS-C18 is a superficially porous particle (SPP) reversed phase column, created by grafting a positive charge to the silica surface and then functionalising the particle with a C18 stationary phase. This chemistry has demonstrated improved peak shapes and enhanced loadability for basic compounds, especially in weak ionic strength mobile phases, for example, containing low concentration formic acid, or low concentration volatile buffers such as ammonium formate. Given the high concentrations of ionisable lipid in modern liquid nanoparticles (in the present formulation, the tertiary amine SM-102 accounts for 50% of the constituent lipids), this stationary phase was deemed an ideal candidate to provide sharp, undistorted peaks.
Chromatographic conditions:
| Column | Poroshell 120 CS-C18, 2.7μm, 2.1 × 100 mm (PN 695775-942) |
| Flow Rate | 0.6 mL/min |
| Column Temperature | 40 °C |
| Detection | ELSD |
| Mobile Phases | A: 5mM ammonium formate in deionised water/ acetonitrile/ methanol (25:35:40) B: 5mM ammonium formate in methanol/ ethanol (60:40) |
| Gradient | Time (min) | %B |
|
0 |
15 | |
| 1 | 15 | |
| 30 | 100 | |
| 35 | 100 | |
| 35.1 | 15 | |
| 42 | 15 |
The lipid components of LNPs are constantly evolving, as formulations are optimised to enhance the potency and efficacy of each RNA-based drug. The most effective way to approach the chemical characterisation of a new formulation is to screen a range of reversed phase columns of complementary selectivities using a generic method. Method conditions are subsequently refined only for the most promising columns, whilst columns exhibiting poor performance during screening are discarded without further work.
Application note 5994-6051EN [4] demonstrates such a screening approach for the four lipid components of the Pfizer-BioNTech Covid 19 vaccine. Using a generic, fast (5 minutes analysis time) ammonium acetate/ methanol gradient, the following six columns were investigated:
- ZORBAX RRHD 300 Å Diphenyl, 1.8μm, 2.1 × 50 mm (part number 857750-944)
- AdvanceBio RP-mAb Diphenyl, 3.5μm, 2.1 × 50 mm (part number 799775-944)
- ZORBAX StableBond 300 CN, 3.5μm, 4.6 × 50 mm (part number 865973-905)
- InfinityLab Poroshell 120 Phenyl-Hexyl, 1.9μm, 2.1 × 50 mm (part number 699675-912)
- AdvanceBio Peptide Plus, 2.7μm, 2.1 × 50 mm (part number 699775-949)
- InfinityLab Poroshell 120 CS-C18, 2.7μm, 2.1 × 50 mm (part number 679775-942)


Figure 3: Column screening results using generic gradient. See text for discussion. Adapted from [4].
As seen in Figure 3, columns number 1 and number 3 show promising results. For column 2, eluent conditions were too strong. The opposite is observed for columns 4, 5, and 6, which show high retention of the analytes under the screening conditions. All columns except 1 were carried over to a second round of tests, which used acetonitrile as a stronger eluent. This study demonstrated that an HPLC system equipped with an automated column switching valve could identify, in a small number of fast screening rounds, the most promising stationary phases. Columns 1 (Zorbax 300Å Diphenyl) and 4 (Poroshell 120 Phenyl-Hexyl) were selected for further method optimisation.


Figure 4: initial (left) and optimised (right) chromatograms of LNP constituent lipids on Poroshell 120 Phenyl-Hexyl, the column that exhibited the best chromatography in the current screening exercise. Adapted from [4].
Element Lab Solutions offer a range of tools designed to facilitate your column screening procedure. These include our free column selection service, underpinned by the Hydrophobic Subtraction Model (HSM2), method development, optimisation, and troubleshooting consultancy, method development kits, an extensive portfolio of loan columns, and Buy & Try purchase options. All these services and products are backed by our best-in-class Tech Support, Tech BD, and Knowledge Transfer teams.
Stability on Agilent Poroshell CS-C18
One of the key therapeutic advantages of LNPs is their ability to protect the payload from endogenous ribonucleases, which would rapidly cleave and degrade unprotected RNA products. It is therefore important to determine the stability of the lipid nanoparticles both in storage and under biological conditions.
During the study reported in [3], LNP components were subjected to forced degradation under acidic, basic, and oxidative conditions. The chromatographic method developed for the quantitative analysis of LNP components was utilised to determine degradation products, demonstrating that stability studies of liquid nanoparticle formulations can be efficiently, quickly, and reliably carried out on the Poroshell CS-C18 column:


Figure 5: Degradation products of ionisable lipid SM-102 (top) and phospholipid DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine, bottom) analysed on Poroshell 120 CS-C18. Adapted from [3].
Particle Size and Size Distribution on Agilent aquagel-OH
Dynamic Light Scattering (DLS) is commonly used to determine the size distribution profile of nanoparticles in solution. DLS analysis, however, can suffer from interferences from proteins and other biological polymers when applied to the analysis of LNPs in plasma samples. In application note 5994-6854EN [5], Agilent Technologies and the Bioprocessing Technology Institute of Singapore demonstrated that size exclusion chromatography (SEC) in line with dual-angle light scattering (LS) and refractive index (RI) detection enabled the simultaneous measurement of hydrodynamic radius, average molecular weight, and semi-quantitative determination of LNP concentration in plasma.
Two Size Exclusion Chromatography (SEC) columns of different pore sizes were connected in series. The first column, PL aquagel-OH Mixed-M, contains a mixture of polymeric particles with different pore sizes. The mixture is optimised to separate biopolymers within a mass range of 500Da to 500kDa. The second column, aquagel-OH Mixed-H, is designed to provide a much higher molecular weight cutoff, up to 10 million Dalton. The two columns were carefully selected to complement each other, enabling the researchers to determine the hydrodynamic radius of lipid nanoparticles over a much wider range than either column could cover individually:


Figure 6: SEC chromatogram overlaying dual-angle light scattering (15° and 90° LS) and refractive index (RI) profiles of LNPs on two PL aquagel-OH columns of different pore sizes and molecular weight cutoff ranges, connected in series. The solid blue line and dark red markers across the apex of the peak of interest indicate the calculated molecular weight and hydrodynamic radius of the lipid nanoparticles, which proved to be remarkably monodispersed. Adapted from [5].
Chromatographic conditions:
| Columns |
PL aquagel-OH Mixed-H 8μm 4.6 × 250 mm (PN PL1549-5800, MW cutoff 10MDa) PL aquagel-OH Mixed-M 8μm 4.6 × 250 mm (PN PL1549-5801, MW cutoff 500kDa) in series |
| Mobile Phase | 50mM sodium phosphate pH 7.2 |
| Flow Rate | 0.6 mL/min |
| Column Temperature | 30 °C |
| Detection | DLS and RI |
LNP Degradation and mRNA Release Kinetics
In the same study, mRNA-LNPs were incubated in human plasma and analysed at 15-minute intervals. The analysis showed that the mRNA-LNP peak (encapsulated mRNA) decreased in intensity over time, whilst the peak corresponding to plasma components in solution increased in intensity. This shift was associated with a decrease in molecular weight and hydrodynamic radius, measured by DLS, of the mRNA-LNP particles. This observation is consistent with degradation of the lipid nanoparticles and gradual delivery of the RNA payload, providing a practical method for measuring the drug release kinetics of the LNPs.


Figure 7: Consecutive injections (15-minute intervals) of mRNA-LNPs incubated in human plasma. The overlaid LS (90° light scattering) chromatograms show a reduction in the concentration of intact LNPs and concomitant increase in the concentration of plasma components (plasma proteins, released mRNA, degraded LNPs). Adapted from [5].
Conclusions
Lipid nanoparticles have gathered enormous interest as novel drug delivery and controlled release therapeutic vehicles, especially for oligonucleotide formulations. Several critical quality attributes must be assessed prior to market application of these sophisticated drug products. Element Lab Solutions, in collaboration with Agilent Technologies, provides an extensive portfolio of chromatographic solutions for the chemical and physical characterisation of LNPs, including chemical composition, particle size and polydispersity, degradation studies, and mRNA release kinetics.
References
[1] R. Cross, “Without these lipid shells, there would be no mRNA vaccines for Covid-19,” Chemical & Engineering News, vol. 99, no. 8, p. 36, 2021.
[2] Food and Drug Administration, “Drug Products, Including Biological Products, that Contain Nanomaterials. Guidance for Industry,” U.S. Department of Health and Human Services, 2022.
[3] C.-Y. Ryu et al., Composition Analysis of Lipid Nanoparticle Components with Agilent 1290 Infinity II ELSD, Agilent Technologies, 2023.
[4] S. Schneider, Easy Column Screening for Lipid Nanoparticle Component Analysis, Agilent Technologies, 2024.
[5] B. Liau et al., Analysis of mRNA-Lipid Nanoparticles in Human Plasma using SEC-Static Dual-Angle Light Scattering and Refractive Index Detection, Agilent Technologies, 2024.